Neurofilament
| NF-L low molecular weight neurofilament protein | |
|---|---|
| Identifiers | |
| Symbol | NEFL |
| Entrez | 4747 |
| HUGO | 7739 |
| OMIM | 162280 |
| RefSeq | NM_006158 |
| UniProt | P07196 |
| Other data | |
| Locus | Chr. 8 p21 |
| NF-M medium molecular weight neurofilament protein | |
|---|---|
| Identifiers | |
| Symbol | NEFM |
| Alt. symbols | NEF3 |
| Entrez | 4741 |
| HUGO | 7734 |
| OMIM | 162250 |
| RefSeq | NM_005382 |
| UniProt | P07197 |
| Other data | |
| Locus | Chr. 8 p21 |
| NF-H high molecular weight neurofilament protein | |
|---|---|
| Identifiers | |
| Symbol | NEFH |
| Entrez | 4744 |
| HUGO | 7737 |
| OMIM | 162230 |
| RefSeq | NM_021076 |
| UniProt | P12036 |
| Other data | |
| Locus | Chr. 22 q12.1-13.1 |
| Alpha-internexin neuronal intermediate filament protein | |
|---|---|
| Identifiers | |
| Symbol | INA |
| Alt. symbols | NEF5 |
| Entrez | 9118 |
| HUGO | 6057 |
| OMIM | 605338 |
| RefSeq | NM_032727 |
| UniProt | Q5SYD2 |
| Other data | |
| Locus | Chr. 10 q24 |
| Peripherin neuronal intermediate filament protein | |
|---|---|
| Identifiers | |
| Symbol | PRPH |
| Alt. symbols | NEF4 |
| Entrez | 5630 |
| HUGO | 9461 |
| OMIM | 170710 |
| RefSeq | NM_006262.3 |
| UniProt | P41219 |
| Other data | |
| Locus | Chr. 12 q13.12 |
| Nestin neuronal stem cell intermediate filament protein | |
|---|---|
| Identifiers | |
| Symbol | NES |
| Entrez | 10763 |
| HUGO | 7756 |
| OMIM | 600915 |
| RefSeq | NP_006608 |
| UniProt | P48681 |
| Other data | |
| Locus | Chr. 1 q23.1 |
Neurofilaments (NF) are intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring approximately 10 nm in diameter and many micrometers in length. Together with microtubules and microfilaments, they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into 6 classes based on their gene organization and protein structure. Class I and II are the keratins which are expressed in epithelia. Class III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Note that the neuronal intermediate filament protein peripherin, which was named by Portier and colleagues in 1983,[1] should not be confused with another protein of the same name (also known as peripherin-2 or peripherin-2/rds) that is expressed in the retina. Class IV consists of the neurofilament proteins L, M, H and internexin. Class V consists of the nuclear lamins, and Class VI consists of the protein nestin. The class IV intermediate filament genes all share two unique introns not found in other intermediate filament gene sequences, suggesting a common evolutionary origin from one primitive class IV gene. The term neurofibril is an antiquated term that refers to the fibrous appearance of bundles of neurofilaments in nerve cells when observed in histologically stained tissue sections.[2]
Neurofilament proteins
The protein composition of neurofilaments varies widely across different animal phyla. Most is known about mammalian neurofilaments. Historically, mammalian neurofilaments were originally thought to be composed of just three proteins called neurofilament protein L (low molecular weight; NFL), M (medium molecular weight; NFM) and H (high molecular weight; NFH). These proteins were discovered from studies of axonal transport and are often referred to as the "neurofilament triplet".[3] However, it is now clear that neurofilaments in the mammalian nervous system also contain the protein internexin[4] and that neurofilaments in the peripheral nervous system can also contain the protein peripherin.[5] Thus mammalian neurofilaments are heteropolymers of up to five different proteins: NFL, NFM, NFH, internexin and peripherin and it is incorrect to consider neurofilaments as being composed of just the neurofilament triplet proteins. Moreover, it is clear that the five neurofilament proteins can coassemble in different combinations and with variable stoichiometry in different nerve cell types and at different stages of development. The precise composition of neurofilaments in any given nerve cell depends on the relative expression levels of the neurofilament proteins in that cell at that time. For example, NFH expression is low in developing neurons and increases postnatally in neurons that are myelinated.[6] In the adult nervous system neurofilaments in small unmyelinated axons contain more peripherin and less NFH whereas neurofilaments in large myelinated axons contain more NFH and less peripherin. The Class III intermediate filament subunit, vimentin, is expressed in developing neurons and a few very unusual neurons in the adult in association with Class IV proteins, such as the horizontal neurons of the retina.
| Protein | Amino acids | NCBI Ref Seq | Predicted molecular mass | Apparent molecular mass (SDS-PAGE) |
|---|---|---|---|---|
| Peripherin | 470 | NP_006253.2 | 53.7 kDa | ~56 kDa |
| Internexin | 499 | NP_116116.1 | 55.4 kDa | ~66 kDa |
| Neurofilament protein L | 543 | NP_006149.2 | 61.5 kDa | ~70 kDa |
| Neurofilament protein M | 916 | NP_005373.2 | 102.5 kDa | ~160 kDa |
| Neurofilament protein H | 1020 | NP_066554.2 | 111.9 kDA | ~200 kDa |
The triplet proteins are based upon their relative size (low, medium, high). The apparent molecular mass of each protein determined by SDS-PAGE is greater than the mass predicted from the amino sequence. This is due to the anomalous electrophoretic migration of these proteins and is particularly extreme for neurofilament proteins M and H due to their high content of charged amino acids and extensive phosphorylation. All three neurofilament triplet proteins contain long stretches of polypeptide sequence rich in glutamic acid and lysine residues, and NF-M and especially NF-H also contain multiple tandemly repeated serine phosphorylation sites. These sites almost all contain the peptide lysine-serine-proline (KSP), and phosphorylation is normally found on axonal and not dendritic neurofilaments. Human NF-M has 13 of these KSP sites, while human NF-H is expressed from two alleles one of which produces 44 and the other 45 KSP repeats.
Neurofilament assembly and structure
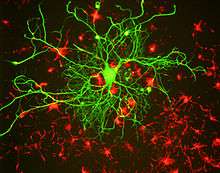
Like other intermediate filament proteins, the neurofilament proteins all share a common central alpha helical region, known as the rod domain because of its rod-like tertiary structure, flanked by amino terminal and carboxy terminal domains that are largely unstructured. The rod domains of two neurofilament proteins dimerize to form an alpha-helical coiled coil. Two dimers associate in a staggered antiparallel manner to form a tetramer. This tetramer is believed to be the basic subunit (i.e. building block) of the neurofilament. Tetramer subunits associate side-to-side to form unit-length filaments, which then anneal end-to-end to form the mature neurofilament polymer, but the precise organization of these subunits within the polymer is not known, largely because of the heterogeneous protein composition and the inability to crystallize neurofilaments or neurofilament proteins. Structural models generally assume eight tetramers (32 neurofilament polypeptides) in a filament cross-section, but measurements of linear mass density suggest that this can vary.
The amino terminal domains of the neurofilament proteins contain numerous phosphorylation sites and appear to be important for subunit interactions during filament assembly. The carboxy terminal domains appear to be intrinsically disordered domains that lack alpha helix or beta sheet. The different sizes of the neurofilament proteins are largely due to differences in the length of the carboxy terminal domains. These domains are rich in acidic and basic amino acid residues. The carboxy terminal domains of NFM and NFH are the longest and are modified extensively by post-translational modifications such as phosphorylation and glycosylation in vivo. They project radially from the filament backbone to form a dense brush border of highly charged and unstructured domains analogous to the bristles on a bottle brush. These entropically flailing domains have been proposed to define a zone of exclusion around each filament, effectively spacing the filaments apart from their neighbors. In this way, the carboxy terminal projections maximize the space-filling properties of the neurofilament polymers. By electron microscopy, these domains appear as projections called sidearms that appear to contact neighboring filaments.
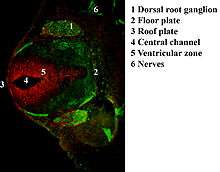
Neurofilament function
Neurofilaments are found in vertebrate neurons in especially high concentrations in axons, where they appear to provide mechanical strength and regulate axonal diameter. The NF-H and NF-M proteins have lengthy C-terminal tail domains that appear to control the spacing between neighboring filaments, generating aligned arrays with a fairly uniform interfilament spacing as seen in axons.
After an axon has grown and connected with its target cell, the diameter of the axon may increase as much as fivefold.
Phosphorylation is thought to contribute to the neurofilament-mediated increase of axonal caliber[7][8] by the binding of divalent cations between the sidearms of adjacent filaments[9][10]
The level of neurofilament gene expression seems to directly control axonal diameter, which in turn controls how fast electrical signals travel down the axon.[11]
Mutant mice with neurofilament abnormalities have phenotypes resembling amyotrophic lateral sclerosis.[12]
Neurofilament transport
In addition to their structural role in axons, neurofilaments are also cargoes of axonal transport.[3] Most of the neurofilament proteins in axons are synthesized in the nerve cell body, where they rapidly assemble into neurofilament polymers within about 30 minutes.[13] These assembled neurofilament polymers are transported along the axon on microtubule tracks powered by microtubule motor proteins.[14] The filaments move bidirectionally, i.e. both towards the axon tip (anterograde) and towards the cell body (retrograde), but the net direction is anterograde. The filaments move at velocities of up to 8 µm/s on short time scales (seconds or minutes), with average velocities of approximately 1 µm/s.[15] However, the average velocity on longer time scales (hours or days) is slow because the movements are very infrequent, consisting of brief sprints interrupted by long pauses.[16][17] Thus on long time scales neurofilaments move in the slow component of axonal transport.
Clinical and research applications
Numerous specific antibodies to neurofilament proteins have been developed and are commercially available. These antibodies can be used to detect neurofilament proteins in cells and tissues using immunofluorescence microscopy or immunohistochemistry. Such antibodies are widely used to identify neurons and their processes in histological sections and in tissue culture. The Class VI intermediate filament protein nestin is expressed in developing neurons and glia. Nestin is considered a marker of neuronal stem cells, and the presence of this protein is widely used to define neurogenesis. This protein is lost as development proceeds.
Neurofilament antibodies are also commonly used in diagnostic neuropathology. Staining with these antibodies can distinguish neurons (positive for neurofilament proteins) from glia (negative for neurofilament proteins).
There is also considerable clinical interest in the use of neurofilament proteins as biomarkers of axonal damage in neurodegenerative diseases. When neurons or axons degenerate, neurofilament proteins are released into the blood or cerebrospinal fluid. Immunoassays of neurofilament proteins in cerebrospinal fluid and plasma can thus serve as indicators of axonal damage in neurological disorders.[18] NFL is a useful marker for disease monitoring in Amyotrophic Lateral Sclerosis,[19] multiple sclerosis[20] and more recently Huntington's disease.[21]
See also
References
- ↑ Portier MM, Brachet P, Croizat B, Gros F (1983). "Regulation of peripherin in mouse neuroblastoma and rat PC 12 pheochromocytoma cell lines". Developmental Neuroscience. 6 (4–5): 215–26. doi:10.1159/000112348. PMID 6151488.
- ↑ Löhrke S, Brandstätter JH, Boycott BB, Peichl L (April 1995). "Expression of neurofilament proteins by horizontal cells in the rabbit retina varies with retinal location". Journal of Neurocytology. 24 (4): 283–300. PMID 7543937.
- 1 2 Hoffman PN, Lasek RJ (August 1975). "The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons". The Journal of Cell Biology. 66 (2): 351–66. PMC 2109569. PMID 49355.
- ↑ Yuan A, Rao MV, Sasaki T, Chen Y, Kumar A, Liem RK, Eyer J, Peterson AC, Julien JP, Nixon RA (September 2006). "Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS". The Journal of Neuroscience. 26 (39): 10006–19. doi:10.1523/jneurosci.2580-06.2006. PMID 17005864.
- ↑ Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, Julien JP, Nixon RA (June 2012). "Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons". The Journal of Neuroscience. 32 (25): 8501–8. doi:10.1523/jneurosci.1081-12.2012. PMC 3405552. PMID 22723690.
- ↑ Nixon RA, Shea TB. "Dynamics of neuronal intermediate filaments: a developmental perspective". Cell Motility and the Cytoskeleton. 22 (2): 81–91. doi:10.1002/cm.970220202. PMID 1633625.
- ↑ Eyer, J.; Leterrier, J. F. (1988). "Influence of the phosphorylation state of neurofilament proteins on the interactions between purified filaments in vitro". Biochem. J. 252: 655–660. doi:10.1042/bj2520655. PMC 1149198.
- ↑ Gou JP, Gotow T, Janmey PA, Leterrier JF (May 1998). "Regulation of neurofilament interactions in vitro by natural and synthetic polypeptides sharing Lys-Ser-Pro sequences with the heavy neurofilament subunit NF-H: neurofilament crossbridging by antiparallel sidearm overlapping". Medical & Biological Engineering & Computing. 36 (3): 371–87. doi:10.1007/BF02522486. PMID 9747580.
- ↑ Kushkuley J, Chan WK, Lee S, Eyer J, Leterrier JF, Letournel F, Shea TB (October 2009). "Neurofilament cross-bridging competes with kinesin-dependent association of neurofilaments with microtubules". Journal of Cell Science. 122 (Pt 19): 3579–86. doi:10.1242/jcs.051318. PMID 19737816.
- ↑ Kushkuley J, Metkar S, Chan WK, Lee S, Shea TB (March 2010). "Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms". Brain Research. 1322: 118–23. doi:10.1016/j.brainres.2010.01.075. PMID 20132798.
- ↑ Alberts B (2002). Molecular biology of the cell (4th ed.). New York: Garland Science. ISBN 0-8153-3218-1. Archived from the original on 2009-10-03.
- ↑ Lalonde R, Strazielle C (2003). "Neurobehavioral characteristics of mice with modified intermediate filament genes". Reviews in the Neurosciences. 14 (4): 369–85. doi:10.1515/REVNEURO.2003.14.4.369. PMID 14640321.
- ↑ Black MM, Keyser P, Sobel E (April 1986). "Interval between the synthesis and assembly of cytoskeletal proteins in cultured neurons". The Journal of Neuroscience. 6 (4): 1004–12. PMID 3084715.
- ↑ Wang L, Ho CL, Sun D, Liem RK, Brown A (March 2000). "Rapid movement of axonal neurofilaments interrupted by prolonged pauses". Nature Cell Biology. 2 (3): 137–41. doi:10.1038/35004008. PMID 10707083.
- ↑ Fenn JD, Johnson CM, Peng J, Jung P, Brown A (January 2018). "Kymograph analysis with high temporal resolution reveals new features of neurofilament transport kinetics". Cytoskeleton. 75 (1): 22–41. doi:10.1002/cm.21411. PMID 28926211.
- ↑ Brown A (November 2000). "Slow axonal transport: stop and go traffic in the axon". Nature Reviews. Molecular Cell Biology. 1 (2): 153–6. doi:10.1038/35040102. PMID 11253369.
- ↑ Brown A, Wang L, Jung P (September 2005). "Stochastic simulation of neurofilament transport in axons: the "stop-and-go" hypothesis". Molecular Biology of the Cell. 16 (9): 4243–55. doi:10.1091/mbc.E05-02-0141. PMC 1196334. PMID 16000374.
- ↑ Jonsson M, Zetterberg H, van Straaten E, Lind K, Syversen S, Edman A, Blennow K, Rosengren L, Pantoni L, Inzitari D, Wallin A (March 2010). "Cerebrospinal fluid biomarkers of white matter lesions - cross-sectional results from the LADIS study". European Journal of Neurology. 17 (3): 377–82. doi:10.1111/j.1468-1331.2009.02808.x. PMID 19845747.
- ↑ Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelsø C (November 1996). "Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF". Journal of Neurochemistry. 67 (5): 2013–8. doi:10.1046/j.1471-4159.1996.67052013.x. PMID 8863508.
- ↑ Teunissen CE, Iacobaeus E, Khademi M, Brundin L, Norgren N, Koel-Simmelink MJ, Schepens M, Bouwman F, Twaalfhoven HA, Blom HJ, Jakobs C, Dijkstra CD (April 2009). "Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis". Neurology. 72 (15): 1322–9. doi:10.1212/wnl.0b013e3181a0fe3f. PMID 19365053.
- ↑ Niemelä V, Landtblom AM, Blennow K, Sundblom J (27 February 2017). "Tau or neurofilament light-Which is the more suitable biomarker for Huntington's disease?". PLOS One. 12 (2): e0172762. doi:10.1371/journal.pone.0172762. PMC 5328385. PMID 28241046.