Aluminium fluoride
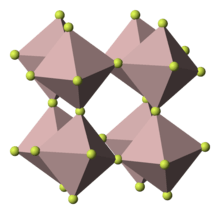 Crystal structure | |
| Names | |
|---|---|
| Other names
Aluminium(III) fluoride Aluminum trifluoride | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.029.137 |
PubChem CID |
|
| RTECS number | BD0725000 |
| |
| |
| Properties | |
| AlF3 | |
| Molar mass | 83.977 g/mol (anhydrous) 101.992 g/mol (monohydrate) 138.023 (trihydrate)[1] |
| Appearance | white, crystalline solid odorless |
| Density | 3.10 g/cm3 (anhydrous) 2.17 g/cm3 (monohydrate) 1.914 g/cm3 (trihydrate)[1] |
| Melting point | 1,290 °C (2,350 °F; 1,560 K)[2] (anhydrous) (sublimes) |
| 5.6 g/L (0 °C) 6.7 g/L (20 °C) 17.2 g/L (100 °C) | |
| -13.4·10−6 cm3/mol[3] | |
Refractive index (nD) |
1.3767 (visible range)[4] |
| Structure | |
| Rhombohedral, hR24 | |
| R3c, No. 167[5] | |
a = 0.49254 nm, c = 1.24477 nm | |
Lattice volume (V) |
0.261519 |
Formula units (Z) |
6 |
| Thermochemistry | |
Heat capacity (C) |
75.1 J/mol·K[6] |
Std molar entropy (S |
66.5 J/mol·K[6] |
Std enthalpy of formation (ΔfH |
−1510.4 kJ/mol[6] |
Gibbs free energy (ΔfG˚) |
-1431.1 kJ/mol[6] |
| Hazards[7][8][9] | |
| Safety data sheet | InChem MSDS |
| GHS pictograms |     |
| GHS signal word | DANGER |
| H301, H302, H314, H315, H319, H335, H361, H372 | |
| P260, P261, P264, P270, P271, P280, P301+310, P301+312, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
| NFPA 704 | |
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
none |
REL (Recommended) |
2 mg/m3 |
IDLH (Immediate danger) |
N.D. |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aluminium fluoride (AlF3) is an inorganic compound used primarily in the production of aluminium. This colorless solid can be prepared synthetically but also occurs in nature as minerals rosenbergite and oskarssonite.
Production and occurrence
The majority of aluminium fluoride is produced by treating alumina with hydrogen fluoride gas at 700 °C:[2] Fluorosilicic acid may also be used make aluminum fluoride.[10]
- H2SiF6 + Al2O3 .3 H2O → 2 AlF3 + 3 SiO2 + 4 H2O
Alternatively, it is manufactured by thermal decomposition of ammonium hexafluoroaluminate.[11] For small scale laboratory preparations, AlF3 can also be prepared by treating aluminium hydroxide or aluminium metal with hydrogen fluoride.
Aluminium fluoride trihydrate is found in nature as the rare mineral rosenbergite. The non-hydrated form appears as the mineral oskarssonite.[12]
Structure
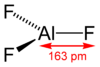
Its structure adopts the rhenium trioxide motif, featuring distorted AlF6 octahedra. Each fluoride is connected to two Al centers. Because of its 3-dimensional polymeric structure, AlF3 has a high melting point. The other trihalides of aluminium in the solid state differ, AlCl3 has a layer structure and AlBr3 and AlI3, are molecular dimers.[13] Also they have low melting points and evaporate readily to give dimers.[14] In the gas phase aluminium fluoride exists as trigonal molecules of D3h symmetry. The Al-F bond lengths of this gaseous molecule are 163 pm.
Applications
Aluminium fluoride is an important additive for the production of aluminium by electrolysis.[2] Together with cryolite, it lowers the melting point to below 1000 °C and increases the conductivity of the solution. It is into this molten salt that aluminium oxide is dissolved and then electrolyzed to give bulk Al metal.[11]
Aluminum fluoride complexes are used to study the mechanistic aspects of phosphoryl transfer reactions in biology, which are of fundamental importance to cells, as phosphoric acid anhydrides such as ATP and GTP control most of the reactions involved in metabolism, growth and differentiation.[15] The observation that aluminum fluoride can bind to and activate heterotrimeric G proteins has proven to be useful for the study of G protein activation in vivo, for the elucidation of three-dimensional structures of several GTPases, and for understanding the biochemical mechanism of GTP hydrolysis, including the role of GTPase-activating proteins.[16]
Niche uses
Together with zirconium fluoride, aluminium fluoride is an ingredient for the production of fluoroaluminate glasses.
It is also used to inhibit fermentation.
Like magnesium fluoride it used as a low-index optical thin film, particularly when far UV transparency is required. Its deposition by physical vapor deposition, particularly by evaporation, is favorable.
Safety
Aluminum fluoride reported oral animal lethal dose (LD50) is 0.1 g/kg.[17] Repeated or prolonged inhalation exposure may cause asthma, and may have effects on the bone and nervous system, resulting in bone alterations (fluorosis), and nervous system impairment.[18]
Many of the neurotoxic effects of fluoride are due to the formation of aluminum fluoride complexes, which mimic the chemical structure of a phosphate and influence the activity of ATP phosphohydrolases and phospholipase D. Only micromolar concentrations of aluminum are needed to form aluminum fluoride.[19]
Human exposure to aluminum fluoride can occur in an industrial setting, such as emissions from an aluminum reduction processes,[20] or when a person ingests both a fluoride source (e.g., fluoride in drinking water or residue of fluoride-based pesticides) and an aluminum source; sources of human exposure to aluminum include drinking water, tea, food residues, infant formula, aluminum-containing antacids or medications, deodorants, cosmetics, and glassware.[19] Fluoridation chemicals may also contain aluminum fluoride.[21] Data on the potential neurotoxic effects of chronic exposure to the aluminum species existing in water is limited.[22]
References
- 1 2 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.45. ISBN 1439855110.
- 1 2 3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 233. ISBN 0-08-037941-9.
- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.131. ISBN 1439855110.
- ↑ Lide, David R. (2003-06-19). CRC Handbook of Chemistry and Physics, 84th Edition. CRC Handbook. CRC Press. ISBN 9780849304842.
- ↑ Hoppe, R.; Kissel, D. (1984). "Zur kenntnis von AlF3 und InF3 [1]". Journal of Fluorine Chemistry. 24 (3): 327. doi:10.1016/S0022-1139(00)81321-4.
- 1 2 3 4 Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 5.5. ISBN 1439855110.
- ↑ Pohanish, Richard P. (2005-03-04). HazMat Data: For First Response, Transportation, Storage, and Security. John Wiley & Sons. ISBN 9780471726104.
- ↑ "Aluminum Fluoride". PubChem. National Institute of Health. Retrieved October 12, 2017.
- ↑ "NIOSH Pocket Guide to Chemical Hazards #0024". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Dreveton, Alain (2012-01-01). "Manufacture of Aluminium Fluoride of High Density and Anhydrous Hydrofluoric Acid from Fluosilicic Acid". Procedia Engineering. SYMPHOS 2011 - 1st International Symposium on Innovation andTechnology in the Phosphate Industry. 46 (Supplement C): 255–265. doi:10.1016/j.proeng.2012.09.471.
- 1 2 Aigueperse, J.; Mollard, P.; Devilliers, D.; Chemla, M.; Faron, R.; Romano, R.; Cuer, J. P. (2005) "Fluorine Compounds, Inorganic" in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a11_307
- ↑ Oskarssonite. Mindat.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Wittinghofer, Alfred (1997-11-01). "Signaling mechanistics: Aluminum fluoride for molecule of the year". Current Biology. 7 (11): R682–R685. doi:10.1016/S0960-9822(06)00355-1.
- ↑ Vincent, Sylvie; Brouns, Madeleine; Hart, Matthew J.; Settleman, Jeffrey (1998-03-03). "Evidence for distinct mechanisms of transition state stabilization of GTPases by fluoride". Proceedings of the National Academy of Sciences. 95 (5): 2210–2215. doi:10.1073/pnas.95.5.2210. ISSN 0027-8424. PMC 19296. PMID 9482864.
- ↑ "ALUMINUM FLUORIDE, CASRN: 7784-18-1". National Library of Medicine HSDB Database. CDC.gov. June 24, 2005. Retrieved October 12, 2017.
- ↑ "ALUMINIUM FLUORIDE (ANHYDROUS) International Chemical Safety Cards (ICSC)". CDC.gov National Institute for Occupational Safety and Health (NIOSH). July 22, 2015. Retrieved July 17, 2017.
- 1 2 Fluoride in Drinking Water: A Scientific Review of EPA's Standards. https://www.nap.edu/read/11571: The National Academies Press. 2006. pp. 51–52, 219. doi:10.17226/11571.
- ↑ TOXICOLOGICAL PROFILE FOR FLUORIDES, HYDROGEN FLUORIDE, AND FLUORINE. https://www.atsdr.cdc.gov/toxprofiles/tp11.pdf: U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry. 2003. p. 211.
- ↑ Mullenix, Phyllis J (2014). "A new perspective on metals and other contaminants in fluoridation chemicals". International Journal of Occupational and Environmental health. 20 (2): 157–166. doi:10.1179/2049396714Y.0000000062. ISSN 1077-3525. PMC 4090869. PMID 24999851.
- ↑ Aluminum Compounds Review of Toxicological Literature Abridged Final Report. Prepared for National Institute of Environmental Health Sciences. NTP.gov Nomination Summary for Aluminum contaminants of drinking water (N20025). October 2001
External links
| Wikimedia Commons has media related to Aluminium fluoride. |
