Cobalt(II) oxide
 | |
| Names | |
|---|---|
| IUPAC name
Cobalt(II) oxide | |
| Other names
Cobaltous oxide Cobalt monoxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.777 |
| EC Number | 215-154-6 |
PubChem CID |
|
| RTECS number | GG2800000 |
| UN number | 3288 |
| |
| |
| Properties | |
| CoO | |
| Molar mass | 74.9326 g/mol |
| Appearance | black powder |
| Odor | odorless |
| Density | 6.44 g/cm3 [1] |
| Melting point | 1,933 °C (3,511 °F; 2,206 K) |
| insoluble in water[2] | |
| +4900.0·10−6 cm3/mol | |
| Structure | |
| cubic, cF8 | |
| Fm3m, No. 225 | |
| Hazards | |
| Main hazards | Toxic (T) Harmful (Xn) |
| Safety data sheet | ICSC 1551 |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R22, R43, R50/53 |
| S-phrases (outdated) | (S2), S24, S37, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
202 mg/kg |
| Related compounds | |
Other anions |
Cobalt(II) sulfide Cobalt(II) hydroxide |
Other cations |
Iron(II) oxide Nickel(II) oxide |
Related compounds |
Cobalt(II,III) oxide Cobalt(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cobalt(II) oxide or cobalt monoxide is an inorganic compound that appears as olive-green to red crystals, or as a greyish or black powder.[3] It is used extensively in the ceramics industry as an additive to create blue colored glazes and enamels as well as in the chemical industry for producing cobalt(II) salts.
Structure and properties
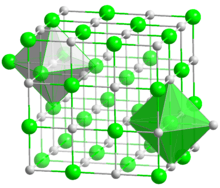
CoO crystals adopt the periclase (rock salt) structure with a lattice constant of 4.2615 Å.[4]
It is antiferromagnetic below 16 °C.[5]
Preparation
Cobalt(II,III) oxide decomposes to cobalt(II) oxide at 950 °C:[6]
- 2 Co3O4 → 6 CoO + O2
Though commercially available, cobalt(II) oxide may be prepared in the laboratory by electrolyzing a solution of cobalt(II) chloride.[7]
CoCl2 + H2O → CoO + H2 + Cl2
It may also be prepared by precipitating the hydroxide, followed by thermal dehydration:
- CoX + 2 KOH → Co(OH)2 + K2X
- Co(OH)2 → CoO + H2O
Reactions
As can be expected, cobalt(II) oxide reacts with mineral acids to form the corresponding cobalt salts:
- CoO + 2 HX → CoX2 + H2O
Applications
Cobalt(II) oxide has for centuries used as a coloring agent on kiln fired pottery. The additive provides a deep shade of blue named cobalt blue. The band gap (CoO) is around 2.4 eV. It also is used in cobalt blue glass.
See also
References
- ↑ Patnaik, Pradyot (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. ISBN 0-07-049439-8. Retrieved 2009-06-06.
- ↑ Advanced Search – Alfa Aesar – A Johnson Matthey Company. Alfa.com. Retrieved on 2011-11-19.
- ↑ "Safety (MSDS) data for cobalt oxide". The Physical and Theoretical Chemistry Laboratory, Oxford University. Retrieved 2008-11-11.
- ↑ Kannan, R.; Seehra, Mohindar S. (1987). "Percolation effects and magnetic properties of the randomly diluted fcc system CopMg1-pO". Physical Review B. 35 (13): 6847–6853. doi:10.1103/PhysRevB.35.6847.
- ↑ Silinsky, P. S.; Seehra, Mohindar S. (1981). "Principal magnetic susceptibilities and uniaxial stress experiments in CoO". Physical Review B. 24: 419–423. doi:10.1103/PhysRevB.24.419.
- ↑ US 4389339, James, Leonard E.; Crescentini, Lamberto & Fisher, William B., "Process for making a cobalt oxide catalyst"
- ↑ Kern, S. (1876). "Inorganic chemistry". J. Chem. Soc. 29: 880. doi:10.1039/JS8762900876.
