Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula C
2H
4 or H2C=CH2. It is a colorless flammable gas with a faint "sweet and musky" odour when pure.[5] It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ethene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.742 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C 2H 4 | |||
| Molar mass | 28.054 g·mol−1 | ||
| Appearance | colorless gas | ||
| Density | 1.178 kg/m3 at 15 °C, gas[1] | ||
| Melting point | −169.2 °C (−272.6 °F; 104.0 K) | ||
| Boiling point | −103.7 °C (−154.7 °F; 169.5 K) | ||
| 0.131 mg/mL (25 °C); 2.9 mg/L[2] | |||
| Solubility in ethanol | 4.22 mg/L[2] | ||
| Solubility in diethyl ether | good[2] | ||
| Acidity (pKa) | 44 | ||
| Conjugate acid | Ethenium | ||
| -15.30·10−6 cm3/mol | |||
| Viscosity | 10.28 μPa·s[3] | ||
| Structure | |||
| D2h | |||
| zero | |||
| Thermochemistry | |||
Std molar entropy (S |
219.32 J·K−1·mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
+52.47 kJ/mol | ||
| Hazards | |||
| Safety data sheet | See: data page ICSC 0475 | ||
EU classification (DSD) (outdated) |
|||
| R-phrases (outdated) | R12 R67 | ||
| S-phrases (outdated) | (S2) S9 S16 S33 S46 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −136 °C (−213 °F; 137 K) | ||
| 542.8 °C (1,009.0 °F; 815.9 K) | |||
| Related compounds | |||
Related compounds |
Ethane Acetylene Propene | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016[6]) exceeds that of any other organic compound.[7][8] Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits.[9] The hydrate of ethylene is ethanol.
Structure and properties

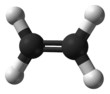
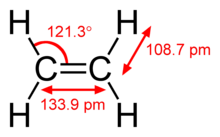
This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively rigid: rotation about the C-C bond is a high energy process that requires breaking the π-bond.
The π-bond in the ethylene molecule is responsible for its useful reactivity. The double bond is a region of high electron density, thus it is susceptible to attack by electrophiles. Many reactions of ethylene are catalyzed by transition metals, which bind transiently to the ethylene using both the π and π* orbitals.
Being a simple molecule, ethylene is spectroscopically simple. Its UV-vis spectrum is still used as a test of theoretical methods.[10]
Uses
Major industrial reactions of ethylene include in order of scale: 1) polymerization, 2) oxidation, 3) halogenation and hydrohalogenation, 4) alkylation, 5) hydration, 6) oligomerization, and 7) hydroformylation. In the United States and Europe, approximately 90% of ethylene is used to produce ethylene oxide, ethylene dichloride, ethylbenzene and polyethylene.[11] Most of the reactions with ethylene are electrophilic addition.

Polymerization
Polyethylene consumes more than half of the world's ethylene supply. Polyethylene, also called polyethene and polythene, is the world's most widely used plastic. It is primarily used to make films in packaging, carrier bags and trash liners. Linear alpha-olefins, produced by oligomerization (formation of short polymers) are used as precursors, detergents, plasticisers, synthetic lubricants, additives, and also as co-monomers in the production of polyethylenes.[11]
Oxidation
Ethylene is oxidized to produce ethylene oxide, a key raw material in the production of surfactants and detergents by ethoxylation. Ethylene oxide is also hydrolyzed to produce ethylene glycol, widely used as an automotive antifreeze as well as higher molecular weight glycols, glycol ethers and polyethylene terephthalate.
Ethylene undergoes oxidation by palladium to give acetaldehyde. This conversion remains a major industrial process (10M kg/y).[12] The process proceeds via the initial complexation of ethylene to a Pd(II) center.
Halogenation and hydrohalogenation
Major intermediates from the halogenation and hydrohalogenation of ethylene include ethylene dichloride, ethyl chloride and ethylene dibromide. The addition of chlorine entails "oxychlorination", i.e. chlorine itself is not used. Some products derived from this group are polyvinyl chloride, trichloroethylene, perchloroethylene, methyl chloroform, polyvinylidene chloride and copolymers, and ethyl bromide.[13]
Alkylation
Major chemical intermediates from the alkylation with ethylene is ethylbenzene, precursor to styrene. Styrene is used principally in polystyrene for packaging and insulation, as well as in styrene-butadiene rubber for tires and footwear. On a smaller scale, ethyltoluene, ethylanilines, 1,4-hexadiene, and aluminium alkyls. Products of these intermediates include polystyrene, unsaturated polyesters and ethylene-propylene terpolymers.[13]
Oxo reaction
The hydroformylation (oxo reaction) of ethylene results in propionaldehyde, a precursor to propionic acid and n-propyl alcohol.[13]
Hydration
Ethylene has long represented the major nonfermentative precursor to ethanol. The original method entailed its conversion to diethyl sulfate, followed by hydrolysis. The main method practiced since the mid-1990s is the direct hydration of ethylene catalyzed by solid acid catalysts:[14]
- C2H4 + H2O → CH3CH2OH
Dimerization to butenes
Ethylene is dimerized by hydrovinylation to give n-butenes using processes licensed by Lummus or IFP. The Lummus process produces mixed n-butenes (primarily 2-butenes) while the IFP process produces 1-butene. 1-Butene is used as a comonomer in the production of certain kinds of polyethylene.
Fruit and flowering
Ethylene is a hormone that affects the ripening and flowering of many plants. It it widely used to control the freshness in horticulture and fruits.
Niche uses
An example of a niche use is as an anesthetic agent (in an 85% ethylene/15% oxygen ratio).[15] Other uses are to hasten the ripening of fruit, and as a welding gas.[11][16]
Production
Global ethylene production was 107 million tonnes in 2005,[7] 109 million tonnes in 2006,[17] 138 million tonnes in 2010, and 141 million tonnes in 2011.[18] By 2013, ethylene was produced by at least 117 companies in 32 countries. To meet the ever-increasing demand for ethylene, sharp increases in production facilities are added globally, particularly in the Mideast and in China.[19]
Industrial process
Ethylene is produced by several methods in the petrochemical industry. A primary method is steam cracking (SC) where hydrocarbons and steam are heated to 750–950 °C. This process converts large hydrocarbons into smaller ones and introduces unsaturation. When ethane is the feedstock, ethylene is the product. Ethylene is separated from the resulting mixture by repeated compression and distillation.[13] In Europe and Asia, ethylene is obtained mainly from cracking naphtha, gasoil and condensates with the coproduction of propylene, C4 olefins and aromatics (pyrolysis gasoline).[20] Other technologies employed for the production of ethylene include oxidative coupling of methane, Fischer-Tropsch synthesis, methanol-to-olefins (MTO), and catalytic dehydrogenation.[21]
Laboratory synthesis
Although of great value industrially, ethylene is rarely synthesized in the laboratory and is ordinarily purchased.[22] It can be produced via dehydration of ethanol with sulfuric acid or in the gas phase with aluminium oxide.[23]
Biosynthesis
Ethylene is produced from methionine in nature. The immediate precursor is 1-aminocyclopropane-1-carboxylic acid.[24]
Ligand

Ethylene is a fundamental ligand in transition metal alkene complexes. One of the first organometallic compounds, Zeise's salt is a complex of ethylene. Useful reagents containing ethylene include Pt(PPh3)2(C2H4) and Rh2Cl2(C2H4)4. The Rh-catalysed hydroformylation of ethylene is conducted on industrial scale to provide propionaldehyde.
History
Some geologists and scholars believe that the famous Greek Oracle at Delphi (the Pythia) went into her trance-like state as an effect of ethylene rising from ground faults.[26]
Ethylene appears to have been discovered by Johann Joachim Becher, who obtained it by heating ethanol with sulfuric acid;[27] he mentioned the gas in his Physica Subterranea (1669).[28] Joseph Priestley also mentions the gas in his Experiments and observations relating to the various branches of natural philosophy: with a continuation of the observations on air (1779), where he reports that Jan Ingenhousz saw ethylene synthesized in the same way by a Mr. Enée in Amsterdam in 1777 and that Ingenhousz subsequently produced the gas himself.[29] The properties of ethylene were studied in 1795 by four Dutch chemists, Johann Rudolph Deimann, Adrien Paets van Troostwyck, Anthoni Lauwerenburgh and Nicolas Bondt, who found that it differed from hydrogen gas and that it contained both carbon and hydrogen.[30] This group also discovered that ethylene could be combined with chlorine to produce the oil of the Dutch chemists, 1,2-dichloroethane; this discovery gave ethylene the name used for it at that time, olefiant gas (oil-making gas.)[31] The term olefiant gas is in turn the etymological origin of the modern word "olefin", the class of hydrocarbons in which ethylene is the first member.
In the mid-19th century, the suffix -ene (an Ancient Greek root added to the end of female names meaning "daughter of") was widely used to refer to a molecule or part thereof that contained one fewer hydrogen atoms than the molecule being modified. Thus, ethylene (C
2H
4) was the "daughter of ethyl" (C
2H
5). The name ethylene was used in this sense as early as 1852.
In 1866, the German chemist August Wilhelm von Hofmann proposed a system of hydrocarbon nomenclature in which the suffixes -ane, -ene, -ine, -one, and -une were used to denote the hydrocarbons with 0, 2, 4, 6, and 8 fewer hydrogens than their parent alkane.[32] In this system, ethylene became ethene. Hofmann's system eventually became the basis for the Geneva nomenclature approved by the International Congress of Chemists in 1892, which remains at the core of the IUPAC nomenclature. However, by that time, the name ethylene was deeply entrenched, and it remains in wide use today, especially in the chemical industry.
Following experimentation by Luckhardt, Crocker, and Carter at the University of Chicago,[33] ethylene was used as an anesthetic.[34][5] It remained in use through the 1940s use even while chloroform was being phased out. Its pungent odor and its explosive nature limit its use today.[35]
Nomenclature
The 1979 IUPAC nomenclature rules made an exception for retaining the non-systematic name ethylene;[36] however, this decision was reversed in the 1993 rules,[37] and it remains unchanged in the newest 2013 recommendations,[38] so the IUPAC name is now ethene. Note that in the IUPAC system, the name ethylene is reserved for the divalent group -CH2CH2-. Hence, names like ethylene oxide and ethylene dibromide are permitted, but the use of the name ethylene for the two-carbon alkene is not. Nevertheless, use of the name ethylene for H2C=CH2 is still prevalent among chemists in North America.
Safety
Like all hydrocarbons, ethylene is an asphyxiant and combustible. It is listed as an IARC class 3 carcinogen as there is no evidence at present that it causes cancer in humans.[39]
References
- Record of Ethylene in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 25 October 2007.
- Neiland, O. Ya. (1990) Органическая химия: Учебник для хим. спец. вузов. Moscow. Vysshaya Shkola. p. 128.
- Kestin J, Khalifa HE, Wakeham WA (1977). "The viscosity of five gaseous hydrocarbons". The Journal of Chemical Physics. 66 (3): 1132–1134. Bibcode:1977JChPh..66.1132K. doi:10.1063/1.434048.
- ETHYLENE | CAMEO Chemicals | NOAA. Cameochemicals.noaa.gov. Retrieved on 2016-04-24.
- Zimmermann H, Walz R (2008). "Ethylene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_045.pub3. ISBN 978-3527306732.
- Research and Markets. "The Ethylene Technology Report 2016 - Research and Markets". www.researchandmarkets.com. Retrieved 19 June 2016.
- "Production: Growth is the Norm". Chemical and Engineering News. 84 (28): 59–236. July 10, 2006. doi:10.1021/cen-v084n034.p059.
- Propylene Production from Methanol. Intratec. 2012-05-31. ISBN 978-0-615-64811-8.
- Wang KL, Li H, Ecker JR (2002). "Ethylene biosynthesis and signaling networks". The Plant Cell. 14 (Suppl): S131-151. doi:10.1105/tpc.001768. PMC 151252. PMID 12045274.
- "Ethylene:UV/Visible Spectrum". NIST Webbook. Retrieved 2006-09-27.
- "OECD SIDS Initial Assessment Profile — Ethylene" (PDF). inchem.org. Archived from the original (PDF) on 2015-09-24. Retrieved 2008-05-21.
- Elschenbroich C, Salzer A (2006). Organometallics: A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-28165-7.
- Kniel L, Winter O, Stork K (1980). Ethylene, keystone to the petrochemical industry. New York: M. Dekker. ISBN 978-0-8247-6914-7.
- Kosaric N, Duvnjak Z, Farkas A, Sahm H, Bringer-Meyer S, Goebel O, Mayer D (2011). "Ethanol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–72. doi:10.1002/14356007.a09_587.pub2. ISBN 9783527306732.
- Trout HH (August 1927). "Blood Changes Under Ethylene Anæsthesia". Annals of Surgery. 86 (2): 260–7. doi:10.1097/00000658-192708000-00013. PMC 1399426. PMID 17865725.
- "Informational Bulletin". 12. California Fresh Market Advisory Board. June 1, 1976. Cite journal requires
|journal=(help) - Nattrass, L and Higson, A (22 July 2010) NNFCC Renewable Chemicals Factsheet: Ethanol. National Non-Food Crops Centre
- True WR (2012). "Global ethylene capacity poised for major expansion". Oil & Gas Journal. 110 (7): 90–95.
- "Market Study: Ethylene (2nd edition), Ceresana, November 2014". ceresana.com. Retrieved 2015-02-03.
- "Ethylene Production and Manufacturing Process". Icis. Retrieved 2019-07-29.
- Amghizar I, Vandewalle LA, Van Geem KM, Marin GB (2017). "New Trends in Olefin Production". Engineering. 3 (2): 171–178. doi:10.1016/J.ENG.2017.02.006.
- Crimmins MT, Kim-Meade AS (2001). "Ethylene". In Paquette, L. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York: Wiley. doi:10.1002/047084289X.re066. ISBN 0471936235.
- Cohen JB (1930). Practical Organic Chemistry (preparation 4). Macmillan.
- Yang SF, Hoffman NE (1984). "Ethylene biosynthesis and its regulation in higher plants". Annu. Rev. Plant Physiol. 35: 155–89. doi:10.1146/annurev.pp.35.060184.001103.
- Neely, Jamie M. (2014). "chlorobis(ethylene)rhodium(I) dimer". e-EROS Encyclopedia of Reagents for Organic Synthesis: 1–6. doi:10.1002/047084289X.rn01715. }}
- Roach J (2001-08-14). "Delphic Oracle's Lips May Have Been Loosened by Gas Vapors". National Geographic. Retrieved March 8, 2007.
- Roscoe HE, Schorlemmer C (1878). A treatise on chemistry. 1. D. Appleton. p. 611.CS1 maint: ref=harv (link)
- Brown JC (July 2006). A History of Chemistry: From the Earliest Times Till the Present Day. Kessinger. p. 225. ISBN 978-1-4286-3831-0.
- Appendix, §VIII, pp. 474 ff., Experiments and observations relating to the various branches of natural philosophy: with a continuation of the observations on air, Joseph Priestley, London: printed for J. Johnson, 1779, vol. 1.
- Roscoe & Schorlemmer 1878, p. 612
- Roscoe & Schorlemmer 1878, p. 613
Gregory W (1857). Handbook of organic chemistry (4th American ed.). A.S. Barnes & Co. p. 157. - Hofmann AW. "Hofmann's Proposal for Systematic Nomenclature of the Hydrocarbons". www.chem.yale.edu. Archived from the original on 2006-09-03. Retrieved 2007-01-06.
- Luckhardt A, Carter JB (1 December 1923). "Ethylene as a gas anesthetic". Current Researches in Anesthesia & Analgesia. 2 (6): 221–229. doi:10.1213/00000539-192312000-00004.
- Johnstone GA (August 1927). "Advantages of Ethylene-Oxygen as a General Anesthetic". California and Western Medicine. 27 (2): 216–8. PMC 1655579. PMID 18740435.
- Whalen FX, Bacon DR, Smith HM (September 2005). "Inhaled anesthetics: an historical overview". Best Practice & Research. Clinical Anaesthesiology. 19 (3): 323–30. doi:10.1016/j.bpa.2005.02.001. PMID 16013684.
- IUPAC nomenclature rule A-3.1 (1979). Acdlabs.com. Retrieved on 2016-04-24.
- Footnote to IUPAC nomenclature rule R-9.1, table 19(b). Acdlabs.com. Retrieved on 2016-04-24.
- Favre, Henri A.; Powell, Warren H., eds. (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. Cambridge: Royal Society of Chemistry. ISBN 9781849733069. OCLC 865143943.
- "Ethylene (IARC Summary & Evaluation, Volume 60, 1994)". www.inchem.org. Retrieved 2019-01-13.
External links
| Wikimedia Commons has media related to Ethylene. |