Indium trihydride
Indium trihydride is an inorganic compound with the chemical formula (InH
3)n (also written as ([InH
3])n or InH
3). It is a covalent network solid, and as such, it is insoluble in all solvents. Moreover, it is unstable at standard temperature and pressure.[2] It is a group 13 hydride.
| Names | |
|---|---|
| Systematic IUPAC name | |
| Other names
Indium(III) hydride Indium trihydride | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 163932 | |
PubChem CID |
|
| |
| |
| Properties | |
| InH 3 | |
| Molar mass | 117.842 g mol−1 |
| Structure | |
| Trigonal planar | |
| Dihedral | |
| Related compounds | |
Related metallanes |
Aluminium hydride Borane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nomenclature
The systematic name indium trihydride, a valid IUPAC name, is constructed according to the compositional nomenclature. Indium trihydride is also used to refer to the related molecular compound indigane and its oligomers. Care should be taken to avoid confusing the two compounds.
Chemical properties
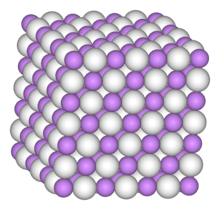
For solid InH3 a three-dimensional network polymeric structure, where In atoms are connected by In-H-In bridging bonds, is suggested to account for the growth of broad infrared bands when samples of InH3 and InD3 produced on a solid hydrogen matrix are warmed.[2] Such a structure is known for solid AlH3.[3] When heated above −90 °C, indium trihydride decomposes to produce indium–hydrogen alloy and elemental hydrogen. As of 2013, the only known method of synthesising indium trihydride is the autopolymerisation of indigane below −90 °C.
Indigane

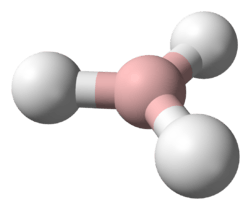
Indigane (also called trihydridoindium or indane, not to be confused with the hydrocarbon indane) is the monomer with the chemical formula InH
3 (also written [InH
3]). It is a colourless gas that cannot persist undiluted. Indigane is the simplest of the indiganes. Unsolvated indigane will spontaneously autopolymerise, first to oligomers, and finally indium trihydride.
It has been observed in matrix isolation.[4][5] Gas phase stability has been predicted.[6] The infrared spectrum was obtained in the gas phase by laser ablation of indium in presence of hydrogen gas [2] Several indium hydride complexes have been reported.[7] Examples of complexes with two hydride ligands replaced by other ligands are K3[K(Me2SiO)7][HIn(Me3CCH2)3]4[8] and HIn(2-Me2NCH2-C6H4)2.
Lewis acidity
A number of indium hydride adducts are known. These include 1:1 adducts with four coordinate indium and 2:1 adducts with five coordinate indium. Typically the 1:1 amine complexes are made by the reaction of indium tribromide with lithium hydride to form LiInH4 in solution which then is reacted with a trialkylammonium chloride. The 2:1 complex with tricyclohexylphosphine (PCy3) may be produced by reacting the 1:1 trimethylammonium complex with PCy3. These adducts are not all stable, for example the trimethylammonium complex is stable only below −30 °C or in dilute solution. The 1:1 and 2:1 complexes with of PCy3 have been characterised crystallographically and the average In-H bond length was found to be 168 pm, which is considerably longer than the corresponding Al–H and Ga–H bonds.[9] Indium hydride is also known to form adducts with carbenes.[10]
The indium atom in indiganes such as InH3 can accept an electron-pair-donating ligand into the molecule by adduction:[11]
- [InH
3] + L → [InH
3L]
Because of this acceptance of the electron-pair donating ligand L, indigane has Lewis-acidic character. Indigane can accept one electron-pair from a ligand, as in the case of the tetrahydridoindate(1−) anion (InH−
4).[12]
References
- "Indigane (CHEBI:30429)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- Andrews, L.; Wang, X. (2004). "Infrared Spectra of Indium Hydrides in Solid Hydrogen and of Solid Indane". Angewandte Chemie International Edition. 43 (13): 1706–1709. doi:10.1002/anie.200353216. PMID 15038043.
- Turley, J. W.; Rinn, H. W. (1969). "The Crystal Structure of Aluminum Hydride". Inorganic Chemistry. 8 (1): 18–22. doi:10.1021/ic50071a005.
- Pullumbi, P.; Bouteiller, Y.; Manceron, L.; Mijoule, C. (July 1994). "Aluminium, gallium and indium trihydrides. An IR matrix isolation and ab initio study". Chemical Physics. 185 (1): 25–37. doi:10.1016/0301-0104(94)00111-1.
- Aldridge, S.; Downs, A. J. (2001). "Hydrides of the Main-Group Metals: New Variations on an Old Theme". Chemical Reviews. 101 (11): 3305–3366. doi:10.1021/cr960151d. PMID 11840988.
- Hunt, P.; Schwerdtfeger, P. (1996). "Are the Compounds InH3 and TlH3 Stable Gas Phase or Solid State Species?". Inorganic Chemistry. 35 (7): 2085–2088. doi:10.1021/ic950411u.
- Hibbs, D. E.; Hursthouse, M. B.; Hursthouse, M. B.; Jones, C.; Smithies, N. A. (1998). "Synthesis, crystal and molecular structure of the first indium trihydride complex, [InH3{(Pri)}]". Chemical Communications (8): 869–870. doi:10.1039/A801118D.
- Rowen Churchill, M.; Lake, C. H.; Chao, S.-H. L.; Beachley, O. T. (1993). "Silicone grease as a precursor to a pseudo crown ether ligand: crystal structure of [K+
]
3[K(Me
2SiO)+
7][InH(CH
2CMe
3)−
3]
4". Journal of the Chemical Society, Chemical Communications. 1993 (20): 1577–1578. doi:10.1039/C39930001577. - Jones, C. (2001). "The stabilisation and reactivity of indium trihydride complexes". Chemical Communications (22): 2293–2298. doi:10.1039/b107285b. ISSN 1359-7345. PMID 12240044.
- Abernethy, C. D.; Cole, M. L.; Jones, C. (2000). "Preparation, Characterization, and Reactivity of the Stable Indium Trihydride Complex [InH3{CN(Mes)C2H2N(Mes)}]". Organometallics. 19 (23): 4852–4857. doi:10.1021/om0004951.
- Wang, X.; Andrews, L. (20 May 2004). "Infrared Spectra of Indium Hydrides in Solid Hydrogen and Neon". The Journal of Physical Chemistry A. 108 (20): 4440–4448. Bibcode:2004JPCA..108.4440W. doi:10.1021/jp037942l.
- Derrah, E. J.; Sircoglou, M.; Mercy, M.; Ladeira, S.; Bouhadir, G.; Miqueu, K.; Maron, L.; Bourissou, D. (28 February 2011). "Original Transition Metal→Indium Interactions upon Coordination of a Triphosphine−Indane". Organometallics. 30 (4): 657–660. doi:10.1021/om1011769.