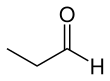
Propionaldehyde
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is a saturated 3-carbon aldehyde and is a structural isomer of acetone. It is a colorless liquid with a slightly irritating, fruity odor.
| |||
 | |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Propanal | |||
| Systematic IUPAC name
Propanal | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.204 | ||
PubChem CID |
|||
| UNII | |||
| UN number | 1275 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.080 g·mol−1 | ||
| Appearance | Colorless liquid Pungent, fruity odor | ||
| Density | 0.81 g cm−3 | ||
| Melting point | −81 °C (−114 °F; 192 K) | ||
| Boiling point | 46 to 50 °C (115 to 122 °F; 319 to 323 K) | ||
| 20 g/100 mL | |||
| -34.32·10−6 cm3/mol | |||
| Viscosity | 0.6 cP at 20 °C | ||
| Structure | |||
| C1, O: sp2
C2, C3: sp3 | |||
| 2.52 D | |||
| Hazards | |||
| GHS pictograms |  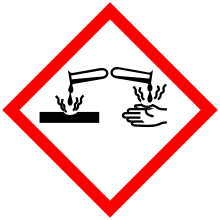  | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H225, H302, H332, H315, H318, H335[1] | ||
| P210, P261, P280, P304+340+312, P305+351+338, P310, P403+235[1] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −26 °C (−15 °F; 247 K) | ||
| 175 °C (347 °F; 448 K) | |||
| Related compounds | |||
Related aldehydes |
Acetaldehyde Butyraldehyde | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Production
Propionaldehyde is mainly produced industrially through hydroformylation, by combining synthesis gas (carbon monoxide and hydrogen) with ethylene using a metal (typically rhodium) catalyst:
- CO + H2 + C2H4 → CH3CH2CHO
In this way, several hundred thousand tons are produced annually.[2]
Laboratory preparation
Propionaldehyde may also be prepared by oxidizing 1-propanol with a mixture of sulfuric acid and potassium dichromate. The reflux condenser contains water heated at 60 °C, which condenses unreacted propanol, but allows propionaldehyde to pass. The propionaldehyde vapor is immediately condensed into a suitable receiver. In this arrangement, any propionaldehyde formed is immediately removed from the reactor, thus it does not get over-oxidized to propionic acid.[3]
Uses
It is principally used as a precursor to trimethylolethane (CH3C(CH2OH)3) through a condensation reaction with formaldehyde; this triol is an important intermediate in the production of alkyd resins. Other applications include reduction to propanol and oxidation to propionic acid.[2]
Condensation of propionaldehyde with tert-butylamine gives CH3CH2CH=N-t-Bu, a three-carbon building block used in organic synthesis. Deprotonation of this imine with LDA produces CH3CHLiCH=N-t-Bu, which in turn condenses with aldehydes.[4]
Extraterrestrial occurrence
Astronomers have detected propionaldehyde (along with acrolein) in the molecular cloud Sagittarius B2 near the center of the Milky Way Galaxy, about 26,000 light years from Earth.[5][6][7]
On 30 July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67/P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which were seen for the first time on a comet, including acetamide, acetone, methyl isocyanate and propionaldehyde.[8][9][10]
References
- Record of Propanal in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 22 March 2020.
- Anthony J. Papa "Propanal" In Ullmann's Encyclopedia of Industrial Chemistry, 2011, WIley-VCH, Weinheim. doi:10.1002/14356007.a22_157.pub2
- Charles D. Hurd and R. N. Meinert (1943). "Propionaldehyde". Organic Syntheses.; Collective Volume, 2, p. 541
- Peralta, M. M. "Propionaldehyde t-Butylimine" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- Scientists Discover Two New Interstellar Molecules: Point to Probable Pathways for Chemical Evolution in Space, National Radio Astronomy Observatory, June 21, 2004
- Two newly found space molecules. By: Goho, Alexandra, Science News, 00368423, 7/24/2004, Vol. 166, Issue 4
- Chemical Precursors to Life Found in Space Scientists say that a universal prebiotic chemistry may be at work
- Jordans, Frank (30 July 2015). "Philae probe finds evidence that comets can be cosmic labs". The Washington Post. Associated Press. Retrieved 30 July 2015.
- "Science on the Surface of a Comet". European Space Agency. 30 July 2015. Retrieved 30 July 2015.
- Bibring, J.-P.; Taylor, M.G.G.T.; Alexander, C.; Auster, U.; Biele, J.; Finzi, A. Ercoli; Goesmann, F.; Klingehoefer, G.; Kofman, W.; Mottola, S.; Seidenstiker, K.J.; Spohn, T.; Wright, I. (31 July 2015). "Philae's First Days on the Comet - Introduction to Special Issue". Science. 349 (6247): 493. Bibcode:2015Sci...349..493B. doi:10.1126/science.aac5116. PMID 26228139.
| Wikimedia Commons has media related to Propionaldehyde. |