Boranes
Boranes is the name given to the class of synthetic hydrides of boron with generic formula BxHy. In the past, borane molecules were often labeled "electron-deficient" because of their multicenter bonding (in which a pair of bonding electrons links more than two atoms, as in 3-center-2-electron bonds); this was done in order to distinguish such molecules from hydrocarbons and other classically bonded compounds. However, this usage is incorrect, as most boranes and related clusters such as carboranes are actually electron-precise, not electron-deficient. For example, the extremely stable icosahedral B12H122- dianion, whose 26 cluster valence electrons exactly fill the 13 bonding molecular orbitals, is in no actual sense deficient in electrons; indeed it is thermodynamically far more stable than benzene.[1]
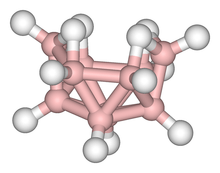
While some boranes are highly reactive with respect to electron-pair donors, others are not, e.g., the BnHn2- dianions (n = 6-12) as well as many neutral boranes such as B18H22. Some lower boranes are pyrophoric in air and react with water. The boranes belong to the class of cluster compounds, which have been the subject of developments in chemical bonding theory. Many of the related anionic hydridoborates have also been synthesized.
History
The development of the chemistry of boranes presented a number of challenges. First, new laboratory techniques had to be developed to handle these often pyrophoric compounds. Alfred Stock created the glass vacuum line, now known as a Schlenk line, for synthesis and handling. The very reactive nature of the lower boranes meant that crystal structure determination was impossible before William Lipscomb developed the requisite techniques. Lastly, once the structures were known it became clear that new theories of chemical bonding were needed to explain them. Lipscomb was awarded the Nobel prize in Chemistry in 1976 for his achievements in this field.
The correct structure of diborane was predicted by H. Christopher Longuet-Higgins[2] 5 years before its determination. Polyhedral skeletal electron pair theory (Wade's rules) can be used to predict the structures of boranes.[3]
Interest in boranes increased during World War II due to the potential of uranium borohydride for enrichment of the uranium isotopes. In the US, a team led by Schlesinger developed the basic chemistry of the boron hydrides and the related aluminium hydrides. Although uranium borohydride was not utilized for isotopic separations, Schlesinger's work laid the foundation for a host of boron hydride reagents for organic synthesis, most of which were developed by his student Herbert C. Brown. Borane-based reagents are now widely used in organic synthesis. Brown was awarded the Nobel prize in Chemistry in 1979 for this work.[4]
Chemical formula and naming conventions
Borane clusters are classified as follows, where n is the number of boron atoms in a single cluster:[5][6]
| Cluster type | Chemical formula | Example | Notes |
|---|---|---|---|
| hypercloso- | BnHn | Unstable; derivatives are known[7] | |
| closo- | BnHn2− | Caesium dodecaborate | |
| nido- | BnHn+4 | pentaborane(9) | |
| arachno- | BnHn+6 | pentaborane(11) | |
| hypho- | BnHn+8 | Only found in adducts |
| Prefix | Meaning | Example |
|---|---|---|
| klado- | branched clusters | |
| conjuncto- | conjoined clusters | |
| megalo- | multiple conjoined clusters |
The International Union of Pure and Applied Chemistry rules for systematic naming is based on a prefix denoting a class of compound, followed by the number of boron atoms and finally the number of hydrogen atoms in parentheses. Various details can be omitted if there is no ambiguity about the meaning, for example, if only one structural type is possible. Some examples of the structures are shown below.
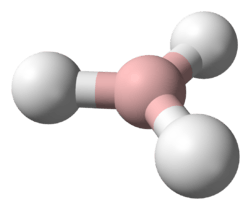 Borane
Borane
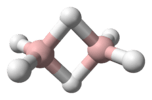
BH3 Diborane(6)
Diborane(6)
B2H6 arachno-Tetraborane(10)
arachno-Tetraborane(10)
B4H10 Pentaborane(9)
Pentaborane(9)
B5H9 Decaborane(14)
Decaborane(14)
B10H14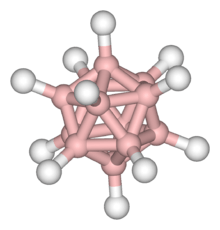 Dodecaborate(12)
Dodecaborate(12)
B12H122− B18H22
B18H22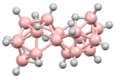 iso-B18H22
iso-B18H22
The naming of anions is illustrated by
- octahydridopentaborate, B5H8−
The hydrogen count is specified first followed by the boron count. The -ate suffix is applied with anions. The ionic charge value is included in the chemical formula but not as not part of the systematic name.
Bonding in boranes
Boranes are nonclassically–bonded compounds, that is, there are not enough electrons to form 2-centre, 2-electron bonds between all pairs of adjacent atoms in the molecule. A description of the bonding in the larger boranes was formulated by William Lipscomb. It involved:
- 3-center 2-electron B-H-B hydrogen bridges
- 3-center 2-electron B-B-B bonds
- 2-center 2-electron bonds (in B-B, B-H and BH2)
Lipscomb's methodology has largely been superseded by a molecular orbital approach. This allows the concept of multi-centre bonding to be extended. For example, in the icosahedral ion [B12H12]2-, the totally symmetric (Ag symmetry) molecular orbital is equally distributed among all 12 boron atoms. Wade's rules provide a powerful method that can be used to rationalize the structures in terms of the number of atoms and the connectivity between them.
There are continuing efforts by theoretical chemists to improve the treatment of the bonding in boranes—an example is Stone's tensor surface harmonic treatment of cluster bonding.[9] A recent development is four-center two-electron bond.
Reactivity of boranes
The lowest borane, BH3, is a very strong Lewis acid. The molecule itself exists only transiently, dimerizing instantly to form diborane, B2H6, but its adducts BH3.THF and BH3.DMSO are stable enough to be used in hydroboration reactions. Other boranes are electrophilic and react vigorously with reagents that can supply electron pairs. With an alkali metal hydride, for example,
- B2H6 + 2 H− → 2 BH4−
Further demonstrating that they are not in general "electron-deficient" (see above), boranes can also function as electron donors owing to the relative basic character of the low-polarity B-Hterminal groups, as in reactions with halogens to form haloboranes.
The reaction of some lower boranes with air is strongly exothermic; those of B2H6 and B5H9, for example, occur explosively except in very low concentration. This does not result from any inherent instability in the boranes. Rather, it is a consequence of the fact that a combustion product, boron trioxide, is a solid. For example
- B2H6(g) + 3 O2(g) → B2O3(s) + 3 H2O(g)
The formation of the solid releases additional energy to what is released by the oxidation reaction. By contrast, many closo-borane anions, such as B12H122-, do not react with air; salts of these anions are metastable because the closo- structure creates a very high activation energy barrier to oxidation.
The higher boranes can be deprotonated when treated with a very strong base. For example,
- B5H9 + NaH → Na(B5H8) + H2
They can also act as weak acids. For example, pentaborane(9) reacts with trimethylphosphine
- B5H9 + 2 PMe3 → B5H9(PMe3)2
producing what can be regarded as a derivative of the unknown hypho-borane B5H13. Acidity increases with the size of the borane.[10] B10H14 has a pK value of 2.7 temperature not stated.
- B5H9 < B6H10 < B10H14 < B16H20 < B18H22
Reaction of a borane with the transient BH3, produced by dissociation of B2H6, can lead to the formation of a conjuncto-borane species in which two small borane sub-units are joined by the sharing of boron atoms.[11]
- B6H10 + (BH3) → B7H11 + H2
- B7H11 + B6H10 → B13H19 + H2
Other conjuncto-boranes, where the sub-units are joined by a B-B bond, can be made by ultra violet irradiation of nido-boranes. Some B-B coupled conjuncto-boranes can be produced using PtBr2 as catalyst.[12]
Reaction of a borane with an alkyne can produce a carborane; the icosahedral closo-carboranes C2B10H12, are particularly stable.[13]
Boranes can function as ligands in coordination compounds.[14] Hapticities of η1 to η6 have been found, with electron donation involving bridging H atoms or donation from B-B bonds. For example, nido-B6H10 can replace ethene in Zeise's salt to produce Fe(η2-B6H10)(CO)4.
Applications
The main chemical application of boranes is the hydroboration reaction. Commercially available adducts such as borane–tetrahydrofuran or borane–dimethylsulfide are often used in this context as they have comparable effectiveness but without the hazard of handling highly reactive BH3 itself.
Neutron capture therapy of cancer is a promising development.[15] The compound used is the HS– (bisulfide) derivative Na2[B12H11(SH)]. It makes use of the fact that 10B has a very high neutron-capture cross section, so neutron irradiation is highly selective for the region where the compound resides.
- 10B + 1n → (11B*) → 4He + 7Li + γ (2.4 Mev)
Boranes have a high specific energy of combustion compared to hydrocarbons, making them potentially attractive as fuels. Intense research was carried out in the 1950s into their use as jet fuel additives, but the effort did not lead to practicable results.
See also
- Category:Boranes, containing all specific borane-compound articles
References
- [1] R. N. Grimes (2016) Carboranes 3rd Edition, Elsevier, New York and Amsterdam, pp. 16-17.
- Longuet-Higgins, H. C.; Bell, R. P. (1943). "64. The Structure of the Boron Hydrides". Journal of the Chemical Society (Resumed). 1943: 250–255. doi:10.1039/JR9430000250.
- Fox, Mark A.; Wade, Ken (2003). "Evolving patterns in boron cluster chemistry" (PDF). Pure Appl. Chem. 75 (9): 1315–1323. doi:10.1351/pac200375091315.
- Brown, H. C. Organic Syntheses via Boranes John Wiley & Sons, Inc. New York: 1975. ISBN 0-471-11280-1.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. pp 151-195
- Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- Peymann, Toralf; Knobler, Carolyn B.; Khan, Saeed I.; Hawthorne, M. Frederick (2001). "Dodeca(benzyloxy)dodecaborane, B12(OCH2Ph)12: A Stable Derivative of hypercloso-B12H12". Angew. Chem. Int. Ed. 40 (9): 1664–1667. doi:10.1002/1521-3773(20010504)40:9<1664::AID-ANIE16640>3.0.CO;2-O.
- Bould, Jonathan; Clegg, William; Teat, Simon J.; Barton, Lawrence; Rath, Nigam P.; Thornton-Pett, Mark; Kennedy, John D. (1999). "An approach to megalo-boranes. Mixed and multiple cluster fusions involving iridaborane and platinaborane cluster compounds. Crystal structure determinations by conventional and synchrotron methods". Inorganica Chimica Acta. 289 (1–2): 95–124. doi:10.1016/S0020-1693(99)00071-7.
- Ceulemans, Arnout; Geert, Mys (1994). "The vector particle of tensor surface harmonic theory". Chemical Physics Letters. 219 (3–4): 274–278. Bibcode:1994CPL...219..274C. doi:10.1016/0009-2614(94)87057-8.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 171
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 162
- Sneddon, L.G. (2009). "Transition metal promoted reactions of polyhedral boranes and carboranes". Pure and Applied Chemistry. 59 (7): 837–846. doi:10.1351/pac198759070837.
- Jemmis, E. D. (1982). "Overlap control and stability of polyhedral molecules. Closo-Carboranes". Journal of the American Chemical Society. 104 (25): 7017–7020. doi:10.1021/ja00389a021.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.p. 177, "The concept of boranes as ligands",
- Sauerwein, Wolfgang; Wittig, Andrea; Moss, Raymond; Nakagawa, Yoshinobu (2012). Neutron Capture Therapy. Berlin: Springer. doi:10.1007/978-3-642-31334-9. ISBN 978-3-642-31333-2.