Hydrogen deuteride
Hydrogen deuteride is a diatomic molecule substance or compound of the two isotopes of hydrogen: the majority isotope 1H (protium) and 2H (deuterium). Its proper molecular formula is H2H, but for simplification, it is usually written as HD.
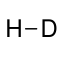 | |
| Names | |
|---|---|
| IUPAC name
Hydrogen deuteride | |
| Systematic IUPAC name
(2H)Dihydrogen | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.325 |
| EC Number |
|
PubChem CID |
|
| UN number | 1049 |
| |
| |
| Properties | |
| H[2H] | |
| Molar mass | 3.02204 g mol−1 |
| Melting point | −259 °C (−434.2 °F; 14.1 K) |
| Boiling point | −253 °C (−423.4 °F; 20.1 K) |
| Hazards | |
EU classification (DSD) (outdated) |
|
| R-phrases (outdated) | R12 |
| S-phrases (outdated) | S16, S33, S36, S38 |
| NFPA 704 (fire diamond) | |
| 571 °C (1,060 °F; 844 K) | |
| Related compounds | |
Related hydrogens |
Deuterium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and occurrence
In the laboratory it is produced by treating sodium hydride with deuterated water:[1]
- NaH + D2O → HD + NaOD
Hydrogen deuteride is a minor component of naturally occurring molecular hydrogen. It is one of the minor but noticeable components of the atmospheres of all the giant planets, with abundances from about 30 ppm to about 200 ppm. HD has also been found in supernova remnants,[2] and other sources.
| Planet | HD | H2 |
|---|---|---|
| Jupiter | ~0.003% | 89.8% ±2.0% |
| Uranus | ~0.007% | 83.0% ±3.0% |
| Neptune | ~0.019% | 80.0% ±3.2% |
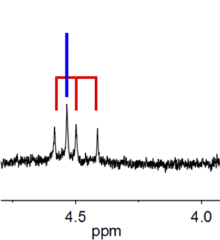
Radio emission spectra
HD and H2 have very similar emission spectra, but the emission frequencies differ.[3]
The frequency of the astronomically important J = 1-0 rotational transition of HD at 2.7 THz has been measured with tunable FIR radiation with an accuracy of 150 kHz.[4]
References
- Bautista, Maria T.; Cappellani, E. Paul; Drouin, Samantha D.; Morris, Robert H.; Schweitzer, Caroline T.; Sella, Andrea; Zubkowski, Jeffery (1991). "Preparation and Spectroscopic Properties of the η2-Dihydrogen Complexes [MH(η2-H2)PR2CH2CH2PR2)2]+ (M = Iron, Ruthenium; R = Ph, Et) and Trends in Properties Down the Iron Group Triad". Journal of the American Chemical Society. 113: 4876–87. doi:10.1021/ja00013a025.CS1 maint: uses authors parameter (link)
- Neufeld, David A.; Hollenbach, David J.; Kaufman, Michael J.; Snell, Ronald L.; Melnick, Gary J.; Bergin, Edwin A.; Sonnentrucker, Paule (2007). "SpitzerSpectral Line Mapping of Supernova Remnants. I. Basic Data and Principal Component Analysis". The Astrophysical Journal. 664 (2): 890. arXiv:0704.2179. Bibcode:2007ApJ...664..890N. doi:10.1086/518857.
- Quinn, W.; Baker, J.; Latourrette, J.; Ramsey, N. (1958). "Radio-Frequency Spectra of Hydrogen Deuteride in Strong Magnetic Fields". Phys. Rev. 112 (6): 1929. Bibcode:1958PhRv..112.1929Q. doi:10.1103/PhysRev.112.1929.
- Evenson, K. M.; Jennings, D. A.; Brown, J. M.; Zink, L. R.; Leopold, K. R. (1988). "Frequency measurement of the J = 1-0 rotational transition of HD". Astrophysical Journal. 330: L135. Bibcode:1988ApJ...330L.135E. doi:10.1086/185221.