Diphosphene
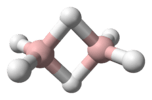
Diphosphene is a compound having the formula (PH)2. It exists as two geometric isomers, E and Z.[1] Diphosphene is also the parent member of the entire class of diphosphene compounds with the formula (PR)2, where R is an organyl group.[2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| P 2H 2 | |
| Molar mass | 63.96340 g·mol−1 |
| Related compounds | |
Other anions |
diazene |
Other cations |
diphosphenes |
Related Binary azenes |
triazene tetrazene |
Related compounds |
ammonia diazane triazane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Lu, T.; Simmonett, A. C.; Evangelista, F. A.; Yamaguchi, Y.; Schaefer, H. F. (2009). "Diphosphene and Diphosphinylidene". The Journal of Physical Chemistry A. 113 (47): 13227–13236. doi:10.1021/jp904028a.
- Yoshifuji, M.; Shibayama, K.; Inamoto, N.; Hirotsu, K.; Higuchi, T. (1983). "Reaction of the diphosphene ArP=PAr (Ar = 2,4,6-But3C6H2) with sulphur: isolation and X-ray structure of the diphosphene monosulphide". Journal of the Chemical Society, Chemical Communications. 1983 (16): 862–863. doi:10.1039/C39830000862.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.