Na+/K+-ATPase
| Na+/K+ ATPase pump | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Sodium-potassium pump, E2-Pi state. Calculated hydrocarbon boundaries of the lipid bilayer are shown as blue (intracellular) and red (extracellular) planes | |||||||||
| Identifiers | |||||||||
| EC number | 3.6.3.9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
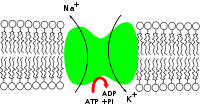
Na+
/K+
-ATPase (sodium-potassium adenosine triphosphatase, also known as the Na+
/K+
pump or sodium–potassium pump) is an enzyme (an electrogenic transmembrane ATPase) found in the plasma membrane of all animal cells. It performs several functions in cell physiology.
The Na+
/K+
-ATPase enzyme is a solute pump that pumps sodium out of cells while pumping potassium into cells, both against their concentration gradients. This pumping is active (i.e. it uses energy from ATP). For every ATP molecule that the pump uses, three sodium ions are exported and two potassium ions are imported; there is hence a net export of a single positive charge per pump cycle.
The sodium-potassium pump was discovered in 1957 by the Danish scientist Jens Christian Skou, who was awarded a Nobel Prize for his work in 1997. Its discovery marked an important step forward in the understanding of how ions get into and out of cells, and it has particular significance for excitable cells such as nerve cells, which depend on this pump to respond to stimuli and transmit impulses.
An alternative theory, Ling’s adsorption theory, posits that the membrane potential and action potential of a living cell is due to the adsorption of mobile ions onto adsorption sites of cells.[1]
All mammals have four different sodium pump sub-types, or isoforms. Each has unique properties and tissue expression patterns.[2]
Function
The Na+
/K+
-ATPase helps maintain resting potential, effect transport, and regulate cellular volume.[3] It also functions as a signal transducer/integrator to regulate MAPK pathway, ROS, as well as intracellular calcium. In most animal cells, the Na+
/K+
-ATPase is responsible for about 1/4 of the cell's energy expenditure. For neurons, the Na+
/K+
-ATPase can be responsible for up to 3/4 of the cell's energy expenditure.[4]
Resting potential

/K+
-ATPase, as well as effects of diffusion of the involved ions maintain the resting potential across the membranes.
In order to maintain the cell membrane potential, cells keep a low concentration of sodium ions and high levels of potassium ions within the cell (intracellular). The sodium-potassium pump mechanism moves 3 sodium ions out and moves 2 potassium ions in, thus, in total, removing one positive charge carrier from the intracellular space (please see Mechanism for details).
Transport
Export of sodium from the cell provides the driving force for several secondary active transporters membrane transport proteins, which import glucose, amino acids, and other nutrients into the cell by use of the sodium gradient.
Another important task of the Na+
-K+
pump is to provide a Na+
gradient that is used by certain carrier processes. In the gut, for example, sodium is transported out of the reabsorbing cell on the blood (interstitial fluid) side via the Na+
-K+
pump, whereas, on the reabsorbing (lumenal) side, the Na+
-glucose symporter uses the created Na+
gradient as a source of energy to import both Na+
and glucose, which is far more efficient than simple diffusion. Similar processes are located in the renal tubular system.
Controlling cell volume
Failure of the Na+
-K+
pumps can result in swelling of the cell. A cell's osmolarity is the sum of the concentrations of the various ion species and many proteins and other organic compounds inside the cell. When this is higher than the osmolarity outside of the cell, water flows into the cell through osmosis. This can cause the cell to swell up and lyse. The Na+
-K+
pump helps to maintain the right concentrations of ions.
Furthermore, when the cell begins to swell, this automatically activates the Na+
-K+
pump.
Functioning as signal transducer
Within the last decade, many independent labs have demonstrated that, in addition to the classical ion transporting, this membrane protein can also relay extracellular ouabain-binding signalling into the cell through regulation of protein tyrosine phosphorylation. The downstream signals through ouabain-triggered protein phosphorylation events include activation of the mitogen-activated protein kinase (MAPK) signal cascades, mitochondrial reactive oxygen species (ROS) production, as well as activation of phospholipase C (PLC) and inositol triphosphate (IP3) receptor (IP3R) in different intracellular compartments.[5]
Protein-protein interactions play a very important role in Na+
-K+
pump-mediated signal transduction. For example, Na+
-K+
pump interacts directly with Src, a non-receptor tyrosine kinase, to form a signaling receptor complex.[6] Src kinase is inhibited by Na+
-K+
pump, while, upon ouabain binding, the Src kinase domain will be released and then activated. Based on this scenario, NaKtide, a peptide Src inhibitor derived from Na+
-K+
pump, was developed as a functional ouabain-Na+
-K+
pump-mediated signal transduction.[7] Na+
-K+
pump also interacts with ankyrin, IP3R, PI3K, PLC-gamma and cofilin.[8]
Controlling neuron activity states
The Na+
-K+
pump has been shown to control and set the intrinsic activity mode of cerebellar Purkinje neurons,[9] accessory olfactory bulb mitral cells[10] and probably other neuron types.[11] This suggests that the pump might not simply be a homeostatic, "housekeeping" molecule for ionic gradients; but could be a computation element in the cerebellum and the brain.[12] Indeed, a mutation in the Na+
-K+
pump causes rapid onset dystonia parkinsonism, which has symptoms to indicate that it is a pathology of cerebellar computation.[13] Furthermore, an ouabain block of Na+
-K+
pumps in the cerebellum of a live mouse results in it displaying ataxia and dystonia.[14] Alcohol inhibits sodium-potassium pumps in the cerebellum and this is likely how it corrupts cerebellar computation and body co-ordination.[15][16] The distribution of the Na+
-K+
pump on myelinated axons, in human brain, was demonstrated to be along the internodal axolemma, and not within the nodal axolemma as previously thought.[17]
Mechanism
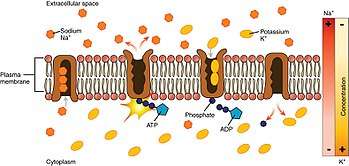
- The pump, after binding ATP, binds 3 intracellular Na+
ions.[3] - ATP is hydrolyzed, leading to phosphorylation of the pump at a highly conserved aspartate residue and subsequent release of ADP.
- A conformational change in the pump exposes the Na+
ions to the outside. The phosphorylated form of the pump has a low affinity for Na+
ions, so they are released. - The pump binds 2 extracellular K+
ions. This causes the dephosphorylation of the pump, reverting it to its previous conformational state, transporting the K+
ions into the cell. - The unphosphorylated form of the pump has a higher affinity for Na+
ions than K+
ions, so the two bound K+
ions are released. ATP binds, and the process starts again.
Regulation
Endogenous
The Na+
/K+
-ATPase is upregulated by cAMP.[18] Thus, substances causing an increase in cAMP upregulate the Na+
/K+
-ATPase. These include the ligands of the Gs-coupled GPCRs. In contrast, substances causing a decrease in cAMP downregulate the Na+
/K+
-ATPase. These include the ligands of the Gi-coupled GPCRs.
Note: Early studies indicated the opposite effect, but these were later found to be inaccurate due to additional complicating factors.
Exogenous
The Na+
-K+
-ATPase can be pharmacologically modified by administrating drugs exogenously.
For instance, Na+
-K+
-ATPase found in the membrane of heart cells is an important target of cardiac glycosides (for example digoxin and ouabain), inotropic drugs used to improve heart performance by increasing its force of contraction.
Muscle contraction is dependent on a 100- to 10,000-times-higher-than-resting intracellular Ca2+
concentration, which is caused by Ca2+
release from the muscle cells' sarcoplasmic reticulum. Immediately after muscle contraction, intracellular Ca2+
is quickly returned to its normal concentration by a carrier enzyme in the plasma membrane, and a calcium pump in sarcoplasmic reticulum, causing the muscle to relax.
Since this carrier enzyme (Na+
-Ca2+
translocator) uses the Na gradient generated by the Na+
-K+
pump to remove Ca2+
from the intracellular space, slowing down the Na+
-K+
pump results in a permanently elevated Ca2+
level in the muscle, which may be the mechanism of the long-term inotropic effect of cardiac glycosides such as digoxin.
Discovery
Na+
/K+
-ATPase was discovered by Jens Christian Skou in 1957 while working as assistant professor at the Department of Physiology, University of Aarhus, Denmark. He published his work that year.[19]
In 1997, he received one-half of the Nobel Prize in Chemistry "for the first discovery of an ion-transporting enzyme, Na+
, K+
-ATPase."[20]
Genes
Additional images
 Mechanism of the sodium-potassium exchange pump.
Mechanism of the sodium-potassium exchange pump.
See also
References
- ↑ Tamagawa H, Funatani M, Ikeda K (January 2016). "Ling's Adsorption Theory as a Mechanism of Membrane Potential Generation Observed in Both Living and Nonliving Systems". Membranes. 6 (1). doi:10.3390/membranes6010011. PMC 4812417. PMID 26821050.
- ↑ Clausen MV, Hilbers F, Poulsen H (June 2017). "The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease". Frontiers in Physiology. 8: 371. doi:10.3389/fphys.2017.00371. PMC 5459889. PMID 28634454.
- 1 2 Hall JE, Guyton AC (2006). Textbook of medical physiology. St. Louis, Mo: Elsevier Saunders. ISBN 0-7216-0240-1.
- ↑ Howarth C, Gleeson P, Attwell D (July 2012). "Updated energy budgets for neural computation in the neocortex and cerebellum". Journal of Cerebral Blood Flow and Metabolism. 32 (7): 1222–32. doi:10.1038/jcbfm.2012.35. PMC 3390818. PMID 22434069.
- ↑ Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z (September 2005). "Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex". Molecular Biology of the Cell. 16 (9): 4034–45. doi:10.1091/mbc.E05-04-0295. PMC 1196317. PMID 15975899.
- ↑ Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ (January 2006). "Binding of Src to Na+/K+-ATPase forms a functional signaling complex". Molecular Biology of the Cell. 17 (1): 317–26. doi:10.1091/mbc.E05-08-0735. PMC 1345669. PMID 16267270.
- ↑ Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z (July 2009). "NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells". The Journal of Biological Chemistry. 284 (31): 21066–76. doi:10.1074/jbc.M109.013821. PMC 2742871. PMID 19506077.
- ↑ Lee K, Jung J, Kim M, Guidotti G (January 2001). "Interaction of the alpha subunit of Na,K-ATPase with cofilin". The Biochemical Journal. 353 (Pt 2): 377–85. doi:10.1042/0264-6021:3530377. PMC 1221581. PMID 11139403.
- ↑ Forrest MD, Wall MJ, Press DA, Feng J (December 2012). "The sodium-potassium pump controls the intrinsic firing of the cerebellar Purkinje neuron". PLOS One. 7 (12): e51169. doi:10.1371/journal.pone.0051169. PMC 3527461. PMID 23284664.
- ↑ Zylbertal A, Kahan A, Ben-Shaul Y, Yarom Y, Wagner S (December 2015). "Prolonged Intracellular Na+ Dynamics Govern Electrical Activity in Accessory Olfactory Bulb Mitral Cells". PLoS Biology. 13 (12): e1002319. doi:10.1371/journal.pbio.1002319. PMID 26674618.
- ↑ Zylbertal A, Yarom Y, Wagner S (2017). "The Slow Dynamics of Intracellular Sodium Concentration Increase the Time Window of Neuronal Integration: A Simulation Study". Frontiers in Computational Neuroscience. 11: 85. doi:10.3389/fncom.2017.00085. PMID 28970791.
- ↑ Forrest MD (December 2014). "The sodium-potassium pump is an information processing element in brain computation". Frontiers in Physiology. 5 (472): 472. doi:10.3389/fphys.2014.00472. PMID 25566080.
- ↑ Cannon SC (July 2004). "Paying the price at the pump: dystonia from mutations in a Na+/K+ -ATPase". Neuron. 43 (2): 153–4. doi:10.1016/j.neuron.2004.07.002. PMID 15260948.
- ↑ Calderon DP, Fremont R, Kraenzlin F, Khodakhah K (March 2011). "The neural substrates of rapid-onset Dystonia-Parkinsonism". Nature Neuroscience. 14 (3): 357–65. doi:10.1038/nn.2753. PMC 3430603. PMID 21297628.
- ↑ Forrest MD (April 2015). "Simulation of alcohol action upon a detailed Purkinje neuron model and a simpler surrogate model that runs >400 times faster". BMC Neuroscience. 16 (27): 27. doi:10.1186/s12868-015-0162-6. PMID 25928094.
- ↑ Forrest M (4 April 2015). "The Neuroscience Reason We Fall Over When Drunk". Science 2.0. Retrieved 30 May 2018.
- ↑ Young EA, Fowler CD, Kidd GJ, Chang A, Rudick R, Fisher E, Trapp BD (April 2008). "Imaging correlates of decreased axonal Na+/K+ ATPase in chronic multiple sclerosis lesions". Annals of Neurology. 63 (4): 428–35. doi:10.1002/ana.21381. PMID 18438950.
- ↑ Burnier, Michel (2008). Sodium In Health And Disease. CRC Press. p. 15. ISBN 978-0-8493-3978-3.
- ↑ Skou JC (February 1957). "The influence of some cations on an adenosine triphosphatase from peripheral nerves". Biochimica et Biophysica Acta. 23 (2): 394–401. doi:10.1016/0006-3002(57)90343-8. PMID 13412736.
- ↑ Chemistry 1997
External links
- Sodium,+Potassium+ATPase at the US National Library of Medicine Medical Subject Headings (MeSH)
- RCSB Protein Data Bank : Sodium-Potassium Pump
- video Khan Academy.