Carboxylic acid
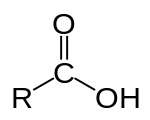
A carboxylic acid /ˌkɑːrbɒkˈsɪlɪk/ is an organic compound that contains a carboxyl group (C(=O)OH).[1] The general formula of a carboxylic acid is R–COOH, with R referring to the rest of the (possibly quite large) molecule. Carboxylic acids occur widely and include the amino acids (which make up proteins) and acetic acid (which is part of vinegar and occurs in metabolism).
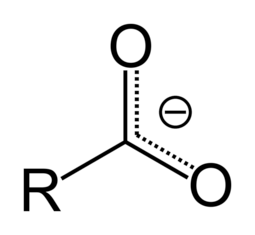
Salts and esters of carboxylic acids are called carboxylates. When a carboxyl group is deprotonated, its conjugate base forms a carboxylate anion. Carboxylate ions are resonance-stabilized, and this increased stability makes carboxylic acids more acidic than alcohols. Carboxylic acids can be seen as reduced or alkylated forms of the Lewis acid carbon dioxide; under some circumstances they can be decarboxylated to yield carbon dioxide.
Example carboxylic acids and nomenclature
Carboxylic acids are commonly identified using their trivial names, and usually have the suffix -ic acid. IUPAC-recommended names also exist; in this system, carboxylic acids have an -oic acid suffix.[2] For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. The -oic acid nomenclature detail is based on the name of the previously-known chemical benzoic acid. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, for example, 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another parent structure, for example, 2-carboxyfuran.
The carboxylate anion (R–COO−) of a carboxylic acid is usually named with the suffix -ate, in keeping with the general pattern of -ic acid and -ate for a conjugate acid and its conjugate base, respectively. For example, the conjugate base of acetic acid is acetate.
| Carbon atoms |
Common name | IUPAC name | Chemical formula | Common location or use |
|---|---|---|---|---|
| 1 | Carbonic acid | Carbonic acid | OHCOOH | Blood and tissues (bicarbonate buffer system) |
| 1 | Formic acid | Methanoic acid | HCOOH | Insect stings |
| 2 | Acetic acid | Ethanoic acid | CH3COOH | Vinegar |
| 3 | Propionic acid | Propanoic acid | CH3CH2COOH | Preservative for stored grains, body odour |
| 4 | Butyric acid | Butanoic acid | CH3(CH2)2COOH | Butter |
| 5 | Valeric acid | Pentanoic acid | CH3(CH2)3COOH | Valerian |
| 6 | Caproic acid | Hexanoic acid | CH3(CH2)4COOH | Goat fat |
| 7 | Enanthic acid | Heptanoic acid | CH3(CH2)5COOH | |
| 8 | Caprylic acid | Octanoic acid | CH3(CH2)6COOH | Coconuts |
| 9 | Pelargonic acid | Nonanoic acid | CH3(CH2)7COOH | Pelargonium |
| 10 | Capric acid | Decanoic acid | CH3(CH2)8COOH | Coconut and Palm kernel oil |
| 11 | Undecylic acid | Undecanoic acid | CH3(CH2)9COOH | |
| 12 | Lauric acid | Dodecanoic acid | CH3(CH2)10COOH | Coconut oil and hand wash soaps |
| 13 | Tridecylic acid | Tridecanoic acid | CH3(CH2)11COOH | |
| 14 | Myristic acid | Tetradecanoic acid | CH3(CH2)12COOH | Nutmeg |
| 15 | Pentadecylic acid | Pentadecanoic acid | CH3(CH2)13COOH | |
| 16 | Palmitic acid | Hexadecanoic acid | CH3(CH2)14COOH | Palm oil |
| 17 | Margaric acid | Heptadecanoic acid | CH3(CH2)15COOH | |
| 18 | Stearic acid | Octadecanoic acid | CH3(CH2)16COOH | Chocolate, waxes, soaps, and oils |
| 19 | Nonadecylic acid | Nonadecanoic acid | CH3(CH2)17COOH | Fats, vegetable oils, pheromone |
| 20 | Arachidic acid | Icosanoic acid | CH3(CH2)18COOH | Peanut oil |
| Compound class | Members |
|---|---|
| unsaturated monocarboxylic acids | acrylic acid (2-propenoic acid) – CH2=CHCOOH, used in polymer synthesis |
| Fatty acids | medium to long-chain saturated and unsaturated monocarboxylic acids, with even number of carbons examples docosahexaenoic acid and eicosapentaenoic acid (nutritional supplements) |
| Amino acids | the building-blocks of proteins |
| Keto acids | acids of biochemical significance that contain a ketone group, e.g. acetoacetic acid and pyruvic acid |
| Aromatic carboxylic acids | benzoic acid, the sodium salt of benzoic acid is used as a food preservative, salicylic acid – a beta hydroxy type found in many skin-care products, phenyl alkanoic acids the class of compounds where a phenyl group is attached to a carboxylic acid. |
| Dicarboxylic acids | containing two carboxyl groups examples adipic acid the monomer used to produce nylon and aldaric acid – a family of sugar acids |
| Tricarboxylic acids | containing three carboxyl groups example citric acid – found in citrus fruits and isocitric acid |
| Alpha hydroxy acids | containing a hydroxy group example glyceric acid, glycolic acid and lactic acid (2-hydroxypropanoic acid) – found in sour milk tartaric acid – found in wine |
| Divinylether fatty acids | containing a doubly unsaturated carbon chain attached via an ether bond to a fatty acid, found in some plants |
Carboxyl radical
The radical •COOH (CAS# 2564-86-5) has only a fleeting isolated existence.[3] The acid dissociation constant of •COOH has been measured using electron paramagnetic resonance spectroscopy.[4] The carboxyl group tends to dimerise to form oxalic acid.
Physical properties
Solubility
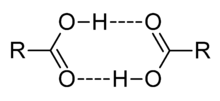
Carboxylic acids are polar. Because they are both hydrogen-bond acceptors (the carbonyl –C=O) and hydrogen-bond donors (the hydroxyl –OH), they also participate in hydrogen bonding. Together the hydroxyl and carbonyl group forms the functional group carboxyl. Carboxylic acids usually exist as dimeric pairs in nonpolar media due to their tendency to "self-associate." Smaller carboxylic acids (1 to 5 carbons) are soluble in water, whereas higher carboxylic acids are less soluble due to the increasing hydrophobic nature of the alkyl chain. These longer chain acids tend to be rather soluble in less-polar solvents such as ethers and alcohols.[5]
Boiling points
Carboxylic acids tend to have higher boiling points than water, not only because of their increased surface area, but also because of their tendency to form stabilised dimers. Carboxylic acids tend to evaporate or boil as these dimers. For boiling to occur, either the dimer bonds must be broken or the entire dimer arrangement must be vaporised, both of which increase the enthalpy of vaporization requirements significantly.
Acidity
Carboxylic acids are Brønsted–Lowry acids because they are proton (H+) donors. They are the most common type of organic acid.
Carboxylic acids are typically weak acids, meaning that they only partially dissociate into H+ cations and RCOO− anions in neutral aqueous solution. For example, at room temperature, in a 1-molar solution of acetic acid, only 0.4% of the acid molecules are dissociated. Electronegative substituents give stronger acids.
| Carboxylic acid[6] | pKa |
|---|---|
| Acetic acid (CH3CO2H) | 4.76 |
| Benzoic acid (C6H5CO2H) | 4.2 |
| Formic acid (HCOOH) | 3.75 |
| Chloroacetic acid (CH2ClCO2H) | 2.86 |
| Dichloroacetic acid (CHCl2CO2H) | 1.29 |
| Oxalic acid (HO2CCO2H) | 1.27 |
| Trichloroacetic acid (CCl3CO2H) | 0.65 |
| Trifluoroacetic acid (CF3CO2H) | 0.23 |
Deprotonation of carboxylic acids gives carboxylate anions; these are resonance stabilized, because the negative charge is delocalized over the two oxygen atoms, increasing the stability of the anion. Each of the carbon–oxygen bonds in the carboxylate anion has a partial double-bond character.
Odor
Carboxylic acids often have strong odors, especially the volatile derivatives. Most common are acetic acid (vinegar) and butyric acid (human vomit). Conversely esters of carboxylic acids tend to have pleasant odors and many are used in perfume.
Characterization
Carboxylic acids are readily identified as such by infrared spectroscopy. They exhibit a sharp band associated with vibration of the C–O vibration bond (νC=O) between 1680 and 1725 cm−1. A characteristic νO–H band appears as a broad peak in the 2500 to 3000 cm−1 region.[5] By 1H NMR spectrometry, the hydroxyl hydrogen appears in the 10–13 ppm region, although it is often either broadened or not observed owing to exchange with traces of water.
Occurrence and applications
Many carboxylic acids are produced industrially on a large scale. They are also pervasive in nature. Esters of fatty acids are the main components of lipids and polyamides of aminocarboxylic acids are the main components of proteins.
Carboxylic acids are used in the production of polymers, pharmaceuticals, solvents, and food additives. Industrially important carboxylic acids include acetic acid (component of vinegar, precursor to solvents and coatings), acrylic and methacrylic acids (precursors to polymers, adhesives), adipic acid (polymers), citric acid (beverages), ethylenediaminetetraacetic acid (chelating agent), fatty acids (coatings), maleic acid (polymers), propionic acid (food preservative), terephthalic acid (polymers).
Synthesis
Industrial routes
In general, industrial routes to carboxylic acids differ from those used on smaller scale because they require specialized equipment.
- Oxidation of aldehydes with air using cobalt and manganese catalysts. The required aldehydes are readily obtained from alkenes by hydroformylation.
- Oxidation of hydrocarbons using air. For simple alkanes, this method is inexpensive but not selective enough to be useful. Allylic and benzylic compounds undergo more selective oxidations. Alkyl groups on a benzene ring are oxidized to the carboxylic acid, regardless of its chain length. Benzoic acid from toluene, terephthalic acid from para-xylene, and phthalic acid from ortho-xylene are illustrative large-scale conversions. Acrylic acid is generated from propene.[7]
- Base-catalyzed dehydrogenation of alcohols.
- Carbonylation is versatile method when coupled to the addition of water. This method is effective for alkenes that generate secondary and tertiary carbocations, e.g. isobutylene to pivalic acid. In the Koch reaction, the addition of water and carbon monoxide to alkenes is catalyzed by strong acids. Acetic acid and formic acid are produced by the carbonylation of methanol, conducted with iodide and alkoxide promoters, respectively, and often with high pressures of carbon monoxide, usually involving additional hydrolytic steps. Hydrocarboxylations involve the simultaneous addition of water and CO. Such reactions are sometimes called "Reppe chemistry":
- HCCH + CO + H2O → CH2=CHCO2H
- Some long-chain carboxylic acids are obtained by the hydrolysis of triglycerides obtained from plant or animal oils; these methods are related to soap making.
- fermentation of ethanol is used in the production of vinegar.
Laboratory methods
Preparative methods for small scale reactions for research or for production of fine chemicals often employ expensive consumable reagents.
- oxidation of primary alcohols or aldehydes with strong oxidants such as potassium dichromate, Jones reagent, potassium permanganate, or sodium chlorite. The method is amenable to laboratory conditions compared to the industrial use of air, which is "greener", since it yields less inorganic side products such as chromium or manganese oxides.
- Oxidative cleavage of olefins by ozonolysis, potassium permanganate, or potassium dichromate.
- Carboxylic acids can also be obtained by the hydrolysis of nitriles, esters, or amides, in general with acid- or base-catalysis.
- Carbonation of a Grignard and organolithium reagents:
- RLi + CO2 → RCO2Li
- RCO2Li + HCl → RCO2H + LiCl
- Halogenation followed by hydrolysis of methyl ketones in the haloform reaction
- The Kolbe–Schmitt reaction provides a route to salicylic acid, precursor to aspirin.
- Base-catalyzed cleavage of non-enolizable ketones, especially aryl ketones:[8]
- RC(O)Ar + H2O → RCO2H + ArH
Less-common reactions
Many reactions afford carboxylic acids but are used only in specific cases or are mainly of academic interest:
- Disproportionation of an aldehyde in the Cannizzaro reaction
- Rearrangement of diketones in the benzilic acid rearrangement involving the generation of benzoic acids are the von Richter reaction from nitrobenzenes and the Kolbe–Schmitt reaction from phenols.
Reactions
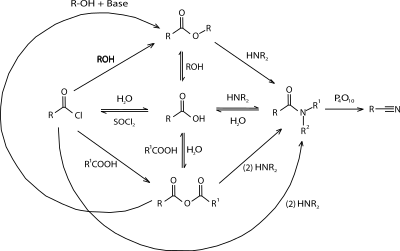
The most widely practiced reactions convert carboxylic acids into esters, amides, carboxylate salts, acid chlorides, and alcohols. Carboxylic acids react with bases to form carboxylate salts, in which the hydrogen of the hydroxyl (–OH) group is replaced with a metal cation. Thus, acetic acid found in vinegar reacts with sodium bicarbonate (baking soda) to form sodium acetate, carbon dioxide, and water:
- CH3COOH + NaHCO3 → CH3COO−Na+ + CO2 + H2O
Carboxylic acids also react with alcohols to give esters. This process is widely used, e.g. in the production of polyesters. Likewise, carboxylic acids are converted into amides, but this conversion typically does not occur by direct reaction of the carboxylic acid and the amine. Instead esters are typical precursors to amides. The conversion of amino acids into peptides is a major biochemical process that requires ATP.
The hydroxyl group on carboxylic acids may be replaced with a chlorine atom using thionyl chloride to give acyl chlorides. In nature, carboxylic acids are converted to thioesters.
Carboxylic acid can be reduced to the alcohol by hydrogenation or using stoichiometric hydride reducing agents such as lithium aluminium hydride.
N,N-Dimethyl(chloromethylene)ammonium chloride (ClHC=N+(CH3)2Cl−) is a highly chemoselective agent for carboxylic acid reduction. It selectively activates the carboxylic acid to give the carboxymethyleneammonium salt, which can be reduced by a mild reductant like lithium tris(t-butoxy)aluminum hydride to afford an aldehyde in a one pot procedure. This procedure is known to tolerate reactive carbonyl functionalities such as ketone as well as moderately reactive ester, olefin, nitrile, and halide moieties.[9]
Specialized reactions
- As with all carbonyl compounds, the protons on the α-carbon are labile due to keto–enol tautomerization. Thus, the α-carbon is easily halogenated in the Hell–Volhard–Zelinsky halogenation.
- The Schmidt reaction converts carboxylic acids to amines.
- Carboxylic acids are decarboxylated in the Hunsdiecker reaction.
- The Dakin–West reaction converts an amino acid to the corresponding amino ketone.
- In the Barbier–Wieland degradation, an carboxylic acid on an aliphatic chain having a simple the methylene bridge at the alpha position can have the chain shortened by one carbon. The inverse procedure is the Arndt–Eistert synthesis, where an acid is converted into acyl halide, which is then reacted with diazomethane to give one additional methylene in the aliphatic chain.
- Many acids undergo oxidative decarboxylation. Enzymes that catalyze these reactions are known as carboxylases (EC 6.4.1) and decarboxylases (EC 4.1.1).
- Carboxylic acids are reduced to aldehydes via the ester and DIBAL, via the acid chloride in the Rosenmund reduction and via the thioester in the Fukuyama reduction.
- In ketonic decarboxylation carboxylic acids are converted to ketones.
- Organolithium reagents (>2 equiv) react with carboxylic acids to give a dilithium 1,1-diolate, a stable tetrahedral intermediate which decomposes to give a ketone upon acidic workup.
- The Kolbe electrolysis is an electrolytic, decarboxylative dimerization reaction. In other words, it gets rid of the carboxyl groups of two acid molecules, and joins the remaining fragments together.
See also
| Wikimedia Commons has media related to Carboxylic acids. |
| Wikiquote has quotations related to: Carboxylic acid |
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "carboxylic acids".
- ↑ Recommendations 1979. Organic Chemistry IUPAC Nomenclature. Rules C-4 Carboxylic Acids and Their Derivatives.
- ↑ Milligan, D. E.; Jacox, M. E. (1971). "Infrared Spectrum and Structure of Intermediates in Reaction of OH with CO". Journal of Chemical Physics. 54 (3): 927–942. Bibcode:1971JChPh..54..927M. doi:10.1063/1.1675022.
- ↑ The value is pKa = −0.2 ± 0.1. Jeevarajan, A. S.; Carmichael, I.; Fessenden, R. W. (1990). "ESR Measurement of the pKa of Carboxyl Radical and Ab Initio Calculation of the Carbon-13 Hyperfine Constant". Journal of Physical Chemistry. 94 (4): 1372–1376. doi:10.1021/j100367a033.
- 1 2 Morrison, R.T.; Boyd, R.N. (1992). Organic Chemistry (6th ed.). ISBN 0-13-643669-2.
- ↑ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. pp. 5–94 to 5–98. ISBN 1439855110.
- ↑ Riemenschneider, Wilhelm (2002). "Carboxylic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a05_235. .
- ↑ Perry C. Reeves (1977). "Carboxylation Of Aromatic Compounds: Ferrocenecarboxylic Acid". Org. Synth. 56: 28. doi:10.15227/orgsyn.056.0028.
- ↑ Fujisawa, Tamotsu; Sato, Toshio. "Reduction of carboxylic acids to aldehydes: 6-Ooxdecanal". Organic Syntheses. 66: 121. doi:10.15227/orgsyn.066.0121. ; Collective Volume, 8, p. 498
External links
| Look up carboxyl in Wiktionary, the free dictionary. |
- Carboxylic acids pH and titration – freeware for calculations, data analysis, simulation, and distribution diagram generation