Fukuyama reduction
| Fukuyama reduction | |
|---|---|
| Named after | Tohru Fukuyama |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | fukuyama-reduction |
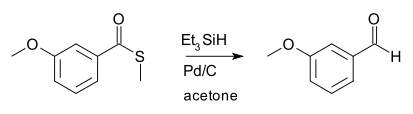
The Fukuyama reduction is an organic reaction and an organic reduction in which a thioester is reduced to an aldehyde by a silyl hydride in presence of a catalytic amount of palladium. This reaction was invented in 1990 by Tohru Fukuyama.[1] In the original scope of the reaction the silyl hydride was triethylsilane and the catalyst palladium on carbon:
Fukuyama reductions are used for the conversion of carboxylic acids (as thioester precursor) to aldehydes which is considered a difficult procedure because of the ease of secondary reduction to an alcohol.
Reaction mechanism
The basic reaction mechanism for this reaction takes place as a catalytic cycle:
Scope
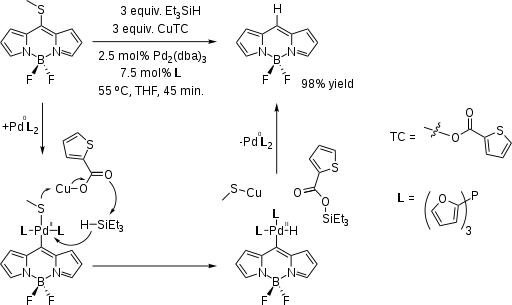
In a variation of the Fukuyama reduction the core BODIPY molecule has been synthesized from the SMe-substituted derivative:[2][3]
In the related Fukuyama coupling the hydride is replaced by a carbon nucleophile.
References
- ↑ Facile reduction of ethyl thiol esters to aldehydes: application to a total synthesis of (+)-neothramycin A methyl ether Tohru Fukuyama, Shao Cheng Lin, Leping Li J. Am. Chem. Soc., 1990, 112 (19), pp 7050–7051 doi:10.1021/ja00175a043
- ↑ The Smallest and One of the Brightest. Efficient Preparation and Optical Description of the Parent Borondipyrromethene System. I. J. Arroyo, R. Hu, G. Merino, B. Z. Tang, E. Peña-Cabrera, J. Org. Chem. 2009, ASAP
- ↑ Additional reagents CuTC, Pd(dba)2, tri(2-furyl)phosphine