Organic peroxide
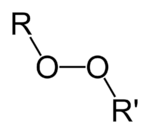
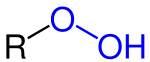

Organic peroxides are organic compounds containing the peroxide functional group (ROOR′). If the R′ is hydrogen, the compounds are called organic hydroperoxides. Peresters have general structure RC(O)OOR. The O−O bond easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds, and this process has been used in explosives. Organic peroxides, like their inorganic counterparts, are powerful bleaching agents.[1]

Properties
The O−O bond length in peroxides is about 1.45 Å, and the R−O−O angles (R = H, C) are about 110° (water-like). Characteristically, the C−O−O−R (R = H, C) dihedral angles are about 120°. The O−O bond is relatively weak, with a bond dissociation energy of 45–50 kcal/mol (190–210 kJ/mol), less than half the strengths of C−C, C−H, and C−O bonds.[2][3]
Major classes of organic peroxides include:
- hydroperoxides, compounds with the functionality ROOH (R = alkyl).
- peroxy acids and esters, compounds with the functionality RC(O)OOH and RC(O)OOR' (R,R' = alkyl, aryl).
- diacyl peroxides, compounds with the functionality RC(O)OOC(O)R (R = alkyl, aryl).
- dialkylperoxides, compounds with the functionality ROOR (R = alkyl).
These compounds occur in nature or are useful in commercial settings. Still other more specialized peroxy compounds are known.[4]
Biology
Peroxides play important roles in biology. Hundreds of peroxides and hydroperoxides are known, being derived from fatty acids, steroids, and terpenes. Derived from fatty acids are a number of 1,2-dioxenes. The biosynthesis prostaglandins proceeds via an endoperoxide, a class of bicyclic peroxides.[5]
In firefly, oxidation of luciferins, which is catalyzed by luciferases, yields a peroxy compound 1,2-dioxetane. The dioxetane is unstable and decays spontaneously to carbon dioxide and excited ketones, which release excess energy by emitting light (bioluminescence).[7]


Industrial uses
In polymer chemistry
Dibenzoyl peroxide is used as a radical initiator, assist polymerization of acrylates. Industrial resins based on acrylic and/or methacrylic acid esters are invariably produced by radical polymerization with organic peroxides at elevated temperatures.[8] The polymerization rate is adjusted by suitable choice of temperature and type of peroxide.[9]
Drying oils, as found in many paints and varnishes function via the formation of hydroperoxides.
Methyl ethyl ketone peroxide, benzoyl peroxide and to a smaller degree acetone peroxide are used as initiators for radical polymerisation of some resins, e.g. polyester and silicone, often encountered when making fiberglass. Pinane hydroperoxide is used in production of styrene-butadiene (synthetic rubber).
Bleaching and disinfecting agents
Benzoyl peroxide and hydrogen peroxide are used as bleaching and "maturing" agents for treating flour to make its grain release gluten more easily; the alternative is letting the flour slowly oxidize by air, which is too slow for the industrialized era. Benzoyl peroxide is an effective topical medication for treating most forms of acne.
In the synthesis of organic compounds
Hydroperoxides are intermediates in the production of many organic compounds in industry. For example, the cobalt catalyzed oxidation of cyclohexane to cyclohexanone:[10]
- C6H12 + O2 → (CH2)5CO + H2O
Acetone and phenol are produced by the so-called cumene process, which proceeds via cumene hydroperoxide.
Many epoxides are prepared using peroxides as reagents, such as the Halcon process that gives propylene oxide. The Sharpless epoxidation is a related reaction conducted on laboratory scale. Tert-butyl hydroperoxide (TBHP) is an organic-soluble oxidant employed in these operations.
Preparation
From hydrogen peroxide
Dialkylsulfates react with alkaline hydrogen peroxide.[11] In this method, the alkyl sulfate donates the alkyl group and the sulfate ion forms the leaving group:
This method can also yield cyclic peroxides.[12] The four-membered dioxetanes can be obtained by 2+2 cycloaddition of oxygen to alkenes.[13]
From O2
The formation of hydroperoxides is effected extensively by enzymes.

This process is used industrially on a very large scale for the production of phenol from benzene and is called the Cumene process or Hock process for its cumene and cumene hydroperoxide intermediates.[14] Here the active site formed by a radical initiator reacts with oxygen to form a hydroperoxyl. The addition of oxygen results in a more active radical which can further extract hydrogen atoms and release the hydroperoxide, leaving a new radical.[15]
In the laboratory, the production of peroxides from O2 is not practiced often. Compounds with allylic and benzylic C−H bonds (see cumene process above) are especially susceptible to oxygenation.[16]
Auto-oxidation reaction is observed with common ethers, such as diethyl ether, diisopropyl ether, tetrahydrofuran or 1,4-dioxane. An illustrative product is diethyl ether peroxide. Such compounds can result in a serious explosion.[15]

Reactions
Hydroperoxides and diorganoperoxides can be reduced to alcohols with lithium aluminium hydride, as described in this idealized equation:
- 4 ROOH + LiAlH4 → LiAlO2 + 2 H2O + 4 ROH
The phosphite esters and tertiary phosphines also effect reduction:
- ROOH + PR3 → OPR3 + ROH
Cleavage to ketones and alcohols in the base catalyzed Kornblum–DeLaMare rearrangement
Some peroxides are drugs, whose action is based on the formation of radicals at desired locations in the organism. For example, artemisinin and its derivatives, such as such artesunate, possess the most rapid action of all current drugs against falciparum malaria.[17] Artesunate is also efficient in reducing egg production in Schistosoma haematobium infection.[18]
Identifying reactions

Several analytical methods are used for qualitative and quantitative determination of peroxides.[19] A simple qualitative detection of peroxides is carried out with the iodine-starch reaction.[20] Here peroxides, hydroperoxides or peracids oxidize the added potassium iodide into iodine, which reacts with starch producing a deep-blue color. Commercial paper indicators using this reaction are available. This method is also suitable for quantitative evaluation, but it can not distinguish between different types of peroxide compounds. Discoloration of various indigo dyes in presence of peroxides is used instead for this purpose.[21] For example, the loss of blue color in leuco-methylene blue is selective for hydrogen peroxide.[22]
Quantitative analysis of hydroperoxides can be performed using potentiometric titration with lithium aluminium hydride.[23] Another way to evaluate the content of peracids and peroxides is the volumetric titration with alkoxides such as sodium ethoxide.[24]
Active oxygen in peroxides
Each peroxy group is considered to contain one active oxygen atom. The concept of active oxygen content is useful for comparing the relative concentration of peroxy groups in formulations, which is related to the energy content. In general, energy content increases with active oxygen content, and thus the higher the molecular weight of the organic groups, the lower the energy content and, usually, the lower the hazard.
The term active oxygen is used to specify the amount of peroxide present in any organic peroxide formulation. One of the oxygen atoms in each peroxide group is considered "active". The theoretical amount of active oxygen can be described by the following equation:[25]
- A[O]theoretical (%) = 16p/m × 100,
where p is the number of peroxide groups in the molecule, and m is the molecular mass of the pure peroxide.
Organic peroxides are often sold as formulations that include one or more phlegmatizing agents. That is, for safety sake or performance benefits the properties of an organic peroxide formulation are commonly modified by the use of additives to phlegmatize (desensitize), stabilize, or otherwise enhance the organic peroxide for commercial use. Commercial formulations occasionally consist of mixtures of organic peroxides, which may or may not be phlegmatized.
Thermal decomposition of organic peroxides
Organic peroxides are useful in chemical synthesis due to their propensity to decompose. In doing so they generate useful radicals that can initiate polymerization to create polymers, modify polymers by grafting or visbreaking, or cross-link polymers to create a thermoset. When used for these purposes, the peroxide is highly diluted, so the heat generated by the exothermic decomposition is safely absorbed by the surrounding medium (e.g. polymer compound or emulsion). But when a peroxide is in a more pure form, the heat evolved by its decomposition may not dissipate as quickly as it is generated, which can result in increasing temperature, which further intensifies the rate of exothermic decomposition. This can create a dangerous situation known as a self-accelerating decomposition.
A self-accelerating decomposition occurs when the rate of peroxide decomposition is sufficient to generate heat at a faster rate than it can be dissipated to the environment. Temperature is the main factor in the rate of decomposition. The lowest temperature at which a packaged organic peroxide will undergo a self-accelerating decomposition within a week is defined as the self-accelerating decomposition temperature (SADT).
Safety

Peroxides are also strong oxidizers and easily react with skin, cotton and wood pulp.[26] For safety reasons, peroxidic compounds are stored in a cool, opaque container, as heating and illumination accelerate their chemical reactions. Small amounts of peroxides, which emerge from storage or reaction vessels are neutralized using reducing agents such as iron(II) sulfate. Safety measures in industrial plants producing large amounts of peroxides include the following:
1) The equipment is located within reinforced concrete structures with foil windows, which would relieve pressure and not shatter in case of explosion.
2) The products are bottled in small containers and are moved to a cold place promptly after the synthesis.
3) The containers are made of non-reactive materials such as stainless steel, some aluminium alloys or dark glass.[27]
For safe handling of concentrated organic peroxides, an important parameter is temperature of the sample, which should be maintained below the self accelerating decomposition temperature of the compound.[28]
The shipping of organic peroxides is restricted. The US Department of Transportation lists organic peroxide shipping restrictions and forbidden materials in 49 CFR 172.101 Hazardous Materials Table based on the concentration and physical state of the material:
| Chemical name | CAS Number | Prohibitions |
|---|---|---|
| Acetyl acetone peroxide | 37187-22-7 | > 9% by mass active oxygen |
| Acetyl benzoyl peroxide | 644-31-5 | solid, or > 40% in solution |
| Ascaridole | 512-85-6 | (organic peroxide) |
| tert-Butyl hydroperoxide | 75-91-2 | > 90% in solution (aqueous) |
| Di-(1-naphthoyl)peroxide | 29903-04-6 | |
| Diacetyl peroxide | 110-22-5 | solid, or > 25% in solution |
| Ethyl hydroperoxide | 3031-74-1 | |
| Iodoxy compounds | dry | |
| Methyl ethyl ketone peroxide | 1338-23-4 | > 9% by mass active oxygen in solution |
| Methyl isobutyl ketone peroxide | 37206-20-5 | > 9% by mass active oxygen in solution |
See also
External links
- OSH Answers – organic peroxides
- "The Perils of Peroxides". carolina.com. Burlington, NC: Carolina Biological Supply Company. Archived from the original on 2007-12-18.
- Peroxide disposal
- Organic Peroxide Producers Safety Division. Oct. 2011. The Society of the Plastics Industry. 24 Oct. 2011.
References
- ↑ Klenk, Herbert; Götz, Peter H.; Siegmeier, Rainer; Mayr, Wilfried, "Peroxy Compounds, Organic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH
- ↑ Bach, Robert D.; Ayala, Philippe Y.; Schlegel, H. B. (1996). "A Reassessment of the Bond Dissociation Energies of Peroxides. An ab Initio Study". J. Am. Chem. Soc. 118: 12758–12765. doi:10.1021/ja961838i.
- ↑ Otto Exner (1983). "Stereochemical and conformational aspects of peroxy compounds". In Saul Patai. PATAI'S Chemistry of Functional Groups. Wiley. pp. 85–96. doi:10.1002/9780470771730.ch2.
- ↑ Saul Patai, ed. (1983). PATAI'S Chemistry of Functional Groups: Peroxides. Wiley. ISBN 9780470771730.
- ↑ D. A. Casteel (1992). "Peroxy Natural Products". Natural Product Reports. 9: 289–312. doi:10.1039/np9920900289.
- ↑ Matsui K (2006). "Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism". Current Opinion in Plant Biology. 9 (3): 274–80. doi:10.1016/j.pbi.2006.03.002. PMID 16595187.
- ↑ Aldo Roda Chemiluminescence and Bioluminescence: Past, Present and Future, p. 57, Royal Society of Chemistry, 2010, ISBN 1-84755-812-7
- ↑ Thomas Brock, Michael Groteklaes, Peter Mischke Lehrbuch der Lacktechnologie, Vincentz Network GmbH & Co KG, 2000, ISBN 3-87870-569-7 p. 67
- ↑ Organische Peroxide für die Polymerisation. pergan.com (in German)
- ↑ Michael T. Musser "Cyclohexanol and Cyclohexanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a08_217
- ↑ Medwedew, S. S.; Alexejewa, E. N. (1932). "Organic peroxides II. Of the reaction between benzoyl hydroperoxide or benzoyl peroxide and triphenylmethyl". Berichte der deutschen chemischen Gesellschaft (A and B Series). 65 (2): 137. doi:10.1002/cber.19320650204.
- ↑ Criegee, Rudolf; Müller, Gerhard (1956). "1.2-Dioxan". Chemische Berichte. 89 (2): 238. doi:10.1002/cber.19560890209.
- ↑ Heinz G. O. Becker Organikum, Wiley-VCH, 2001, ISBN 3-527-29985-8, p. 323
- ↑ Brückner, R. Reaktionsmechanismen: organische Reaktionen, Stereochemie, moderne Synthesemethoden, pp. 41–42, Spektrum Akademischer Verlag, Munich, 2004, ISBN 3-8274-1579-9 (in German)
- 1 2 Heinz G. O. Becker Organikum, Wiley-VCH, 2001, ISBN 3-527-29985-8 pp. 206–207
- ↑ Knight, H. B.; Swern, Daniel (1963). "Tetralin hydroperoxide". Organic Syntheses. ; Collective Volume, 4, p. 895 .
- ↑ White NJ (1997). "Assessment of the pharmacodynamic properties of antimalarial drugs in vivo". Antimicrob. Agents Chemother. 41 (7): 1413–22. PMC 163932. PMID 9210658.
- ↑ Boulangier D, Dieng Y, Cisse B, et al. (2007). "Antischistosomal efficacy of artesunate combination therapies administered as curative treatments for malaria attacks". Trans R Soc Trop Med Hyg. 101 (2): 113–16. doi:10.1016/j.trstmh.2006.03.003. PMID 16765398.
- ↑ Légrádi, L.; Légrádi, J. (1970). "Detection of peroxides, hydroperoxides and peracids". Mikrochimica Acta. 58: 119. doi:10.1007/BF01218105.
- ↑ Lea, C. H. (1931). "The Effect of Light on the Oxidation of Fats". Proceedings of the Royal Society B: Biological Sciences. 108 (756): 175. doi:10.1098/rspb.1931.0030.
- ↑ Veibel, S. Analytik organischer Verbindungen, Akademie-Verlag, Berlin, 1960, p. 262
- ↑ Eiss, M. I.; Giesecke, Paul (1959). "Colorimetric Determination of Organic Peroxides". Analytical Chemistry. 31 (9): 1558. doi:10.1021/ac60153a038.
- ↑ Higuchi, T.; Zuck, Donald Anton (1951). "Behaviors of Several Compounds as Indicators in Lithium Aluminum Hydride Titration of Functional Groups". Journal of the American Chemical Society. 73 (6): 2676. doi:10.1021/ja01150a073.
- ↑ Martin, A. J. (1957). "Potentiometric titration of hydroperoxide and peracid in Anhydrous Ethylenediamine". Analytical Chemistry. 29: 79. doi:10.1021/ac60121a022.
- ↑ "ASTM E298, Standard Test Methods for Assay of Organic Peroxides". ASTM. 2010.
- ↑ Heinz G. O. Becker Organikum, Wiley-VCH, 2001, ISBN 3-527-29985-8 pp. 741–762
- ↑ Ozonelab Peroxide compatibility
- ↑ Organic Peroxide Producers Safety Division (2012-08-06). "Safety and Handling of Organic Peroxides" (PDF). The Society of the Plastics Industry, Inc.