Vinyl group
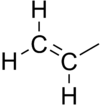
In chemistry, vinyl or ethenyl[1] (abbreviated as Vi[2]) is the functional group with the formula −CH=CH2. It is the ethylene (IUPAC ethene) molecule (H2C=CH2) less one hydrogen atom. The name is also used for any compound containing that group, namely R−CH=CH2 where R is any other group of atoms.
An industrially important example is vinyl chloride, precursor to PVC, a plastic commonly known as vinyl.

Vinyl is one of the alkenyl functional groups. On a carbon skeleton, sp2-hybridized carbons or positions are often called vinylic. Allyls, acrylates and styrenics contain vinyl groups. (A styrenic crosslinker with two vinyl groups is called divinyl benzene.)
Vinyl polymers
Vinyl groups can polymerize with the aid of a radical initiator or a catalyst, forming vinyl polymers. Vinyl polymers contain no vinyl groups. Instead they are saturated. The following table gives some examples of vinyl polymers.
| Monomer example | Example of resulting polymer |
|---|---|
| Vinyl chloride | Polyvinyl chloride (PVC) |
| Vinyl fluoride | Polyvinyl fluoride (PVF) |
| Vinyl acetate | Polyvinyl acetate (PVAc) |
Many vinylidene and vinylene compounds polymerize in the same manner. Those polymers are analogously referred to as polyvinylidenes and polyvinylenes, reflecting the monomeric precursors.
Reactivity
Vinyl derivatives are alkenes. If activated by an adjacent group, the increased polarization of the bond gives rise to characteristic reactivity, which is termed vinylogous:
- In allyl compounds, where the next carbon is saturated but substituted once, allylic rearrangement and related reactions are observed.
- Allyl Grignard reagents (organomagnesiums) can attack with the vinyl end first.
- If next to an electron-withdrawing group, conjugate addition (Michael addition) occurs.
Vinyl organometallics, e.g. vinyl lithium, participate in coupling reactions such as in Negishi coupling.
Etymology
The etymology of vinyl is the Latin vinum = "wine", because of its relationship with alcohol (in its original sense of ethyl alcohol). The term "vinyl" was coined by the German chemist Hermann Kolbe in 1851.[3]