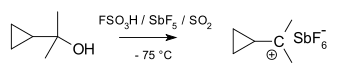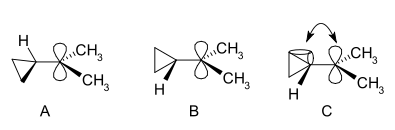Carbocation
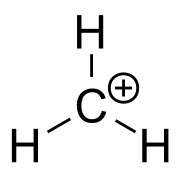
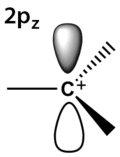

A carbocation (/ˌkɑːrboʊˈkætaɪən/[1] /karbɔkətaɪː'jɔ̃/) is an ion with a positively charged carbon atom. Among the simplest examples are methenium CH+
3, methanium CH+
5, and ethanium C
2H+
7. Some carbocations may have two or more positive charges, on the same carbon atom or on different atoms; such as the ethylene dication C
2H2+
4.[2]
Until the early 1970s, all carbocations were called carbonium ions.[3] In present-day chemistry, a carbocation is any ion with a positively charged carbon atom, classified in two main categories according to the valence of the charged carbon: three in the carbenium ions (protonated carbenes, formally, we can write CH2: + H+ → CH3+), and five or six in the carbonium ions (protonated alkanes, named by analogy to ammonium). This nomenclature was proposed by G. A. Olah.[4] Carbocations are stabilized by the dispersion or delocalization of the positive charge.
Definitions
Some university-level textbooks discuss carbocations as if they were only carbenium ions,[5] or discuss carbocations with only a fleeting reference to the older terminology of carbonium ions[6] or carbenium and carbonium ions.[7] One textbook retains the older name of carbonium ion for carbenium ion to this day, and uses the phrase hypervalent carbenium ion for CH+
5.[8]
History
The history of carbocations dates back to 1891 when G. Merling[9] reported that he added bromine to tropylidene (cycloheptatriene) and then heated the product to obtain a crystalline, water-soluble material, C
7H
7Br. He did not suggest a structure for it; however, Doering and Knox[10] convincingly showed that it was tropylium (cycloheptatrienylium) bromide. This ion is predicted to be aromatic by Hückel's rule.
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol gives deep-yellow solutions in concentrated sulfuric acid. Triphenylmethyl chloride similarly formed orange complexes with aluminium and tin chlorides. In 1902, Adolf von Baeyer recognized the salt-like character of the compounds formed.
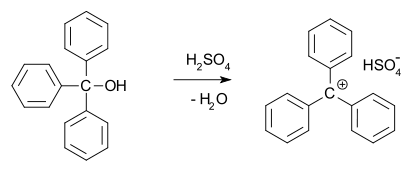
He dubbed the relationship between color and salt formation halochromy, of which malachite green is a prime example.
Carbocations are reactive intermediates in many organic reactions. This idea, first proposed by Julius Stieglitz in 1899,[11] was further developed by Hans Meerwein in his 1922 study[12][13] of the Wagner–Meerwein rearrangement. Carbocations were also found to be involved in the SN1 reaction, the E1 reaction, and in rearrangement reactions such as the Whitmore 1,2 shift. The chemical establishment was reluctant to accept the notion of a carbocation and for a long time the Journal of the American Chemical Society refused articles that mentioned them.
The first NMR spectrum of a stable carbocation in solution was published by Doering et al.[14] in 1958. It was the heptamethylbenzenium ion, made by treating hexamethylbenzene with methyl chloride and aluminium chloride. The stable 7-norbornadienyl cation was prepared by Story et al. in 1960[15] by reacting norbornadienyl chloride with silver tetrafluoroborate in sulfur dioxide at −80 °C. The NMR spectrum established that it was non-classically bridged (the first stable non-classical ion observed).
In 1962, Olah directly observed the tert-butyl carbocation by nuclear magnetic resonance as a stable species on dissolving tert-butyl fluoride in magic acid. The NMR of the norbornyl cation was first reported by Schleyer et al.[16] and it was shown to undergo proton-scrambling over a barrier by Saunders et al.[17]
Structure and properties
Carbonium ions can be thought of as protonated alkanes. Although alkanes are usually considered inert, under superacid conditions (e.g., HF/SbF5), the C-H sigma bond can act as a donor to H+. This results in a species that contains a 3c-2e bond between a carbon and two hydrogen atoms, a type of bonding common in boron chemistry, though relatively uncommon for carbon. As an alternative view point, the 3c-2e bond of carbonium ions could be considered as a molecule of H2 coordinated to a carbenium ion. Indeed, carbonium ions frequently decompose by loss of molecular hydrogen to form the corresponding carbenium ion. Structurally, the methanium ion CH5+ is computed to have a minimum energy structure of Cs symmetry. However, the various possible structures of the ion are close in energy and separated by shallow barriers. Hence, the structure of the ion is often described as fluxional. Although there appear to be five bonds to carbon in carbonium ions, they are not hypervalent, as the electron count around the central carbon is only eight, on account of the 3c-2e bond.
The charged carbon atom in a carbenium ion is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability (octet rule). Therefore, carbocations are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge. In accord with VSEPR and Bent's rule, unless geometrically constrained to be pyramidal (e.g., 1-adamantyl cation), 3-coordinate carbenium ions are usually trigonal planar, with a pure p character empty orbital as its lowest unoccupied molecular orbital and CH/CC bonds formed from C(sp2) orbitals. A prototypical example is the methyl cation, CH+
3. For the same reasons, carbocations that are 2-coordinate (vinyl cations) are generally linear in geometry, with CH/CC bonds formed from C(sp) orbitals.
Alkyl-substituted carbocations follow the order 3° > 2° > 1° > methyl in stability, as can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for (CH3)3C+, (CH3)2CH+, C2H5+, and CH3+).[18] The effect of alkyl substitution is a strong one: tertiary cations are stable and many are directly observable in superacid media, but secondary cations are usually transient and only the isopropyl, s-butyl, and cyclopentyl cations have been observed in solution.[19] Primary cations are seldom encountered in the solution phase, even as transient intermediates, and methyl cation has only been unambiguously identified in the gas phase. In most, if not all cases, the ground state of alleged primary carbocations consist of bridged structures in which positive charge is shared by two or more carbon atoms and are better described as side-protonated alkenes or edge-protonated cyclopropanes rather than true primary cations.[20] Even the simple ethyl cation, C2H5+, has been demonstrated experimentally and computationally to be bridged and can be thought of as a symmetrically protonated ethylene molecule. The same is true for higher homologues like n-propyl cation.[21] Neopentyl derivatives are thought to ionize with concomitant migration of a methyl group (anchimeric assistance); thus, in most if not all cases, a discrete neopentyl cation is not believed to be involved.[22]
The stabilization by alkyl groups is explained by hyperconjugation. The donation of electron density from a β C-H or C-C bond into the unoccupied p orbital of the carbocation (a σCH/CC → p interaction) allows the positive charge to be delocalized.
Based on hydride ion affinity, vinyl cations have a stability similar to that of primary carbocations and are relatively uncommon intermediates. Alkynyl cations are even more unstable and can only be generated by radiochemical means: (RC≡CT → [RC≡C3He]+ + e– → RC≡C+ + 3He + e–).[23]

Carbocations are often the target of nucleophilic attack by nucleophiles like hydroxide (OH−) ions or halogen ions.
Carbocations typically undergo rearrangement reactions from less stable structures to equally stable or more stable ones by migration of an alkyl group or hydrogen to the cationic center to form a new carbocationic center. This often occurs with rate constants in excess of 1010 s−1 at ambient temperature and still takes place rapidly (compared to the NMR timescale) at temperatures as low as –120 °C (see Wagner-Meerwein shift). Typically, carbocations will rearrange to give a tertiary isomer. For instance, all isomers of C6H12+ rapidly rearrange to give the 1-methyl-1-cyclopentyl cation. This fact often complicates synthetic pathways. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce about 1⁄3 3-chloropentane and 2⁄3 2-chloropentane. The Friedel-Crafts alkylation suffers from this limitation; for this reason, the acylation (followed by Wolff-Kishner or Clemmensen reduction to give the alkylated product) is more frequently applied.
A carbocation may be stabilized by resonance by a carbon-carbon double bond next to the ionized carbon. Such cations as allyl cation CH2=CH–CH2+ and benzyl cation C6H5–CH2+ are more stable than most other carbocations due to donation of electron density from π systems to the cationic center. Molecules that can form allyl or benzyl carbocations are especially reactive. These carbocations where the C+ is adjacent to another carbon atom that has a double or triple bond have extra stability because of the overlap of the empty p orbital of the carbocation with the p orbitals of the π bond. This overlap of the orbitals allows the positive charge to be dispersed and electron density from the π system to be shared with the electron-deficient center, resulting in stabilization. For the same reasons, the partial p character of strained C–C bonds in cyclopropyl groups also allows for donation of electron density and stabilizes the cyclopropylmethyl (cyclopropylcarbinyl) cation.
The stability order of carbocation, from most stable to least stable as reflected by hydride ion affinity values, are as follows:
- tropylium ion > triphenylmethyl (trityl) cation > diphenylmethyl cation > tert-butyl carbocation > benzyl > cyclopropylmethyl > allyl
Non-classical ions
Some carbocations such as the 2-norbornyl cation exhibit more or less symmetrical three-center two-electron bonding. Such structures, referred to as non-classical carbocations, involve the delocalization of the bonds involved in the σ-framework of the molecule and the drawing of "no-bond" resonance forms (beyond the relatively simple variety encountered in hyperconjugation). The existence of non-classical carbocations was once the subject of great controversy. On opposing sides were Brown, who believed that the what appeared to be a non-classical carbocation represents the average of two rapidly equilibrating classical species and that the true non-classical structure is a transition state between the two potential energy minima, and Winstein, who believed that the non-classical carbocation was the sole potential energy minimum and that the classical structures merely two contributing resonance forms of this non-classical species. George Olah's discovery of superacidic media to allow carbocations to be directly observed, together with a very sensitive NMR technique developed by Martin Saunders to distinguish between the two scenarios, played important roles in resolving this controversy.[24][25] At least for the 2-norbornyl cation itself, the controversy has been settled overwhelmingly in Winstein's favor, with no sign of the putative interconverting classical species, even at temperatures as low as 6 K, and a 2013 crystal structure showing a distinctly non-classical structure.[26][27] A variety of carbocations (e.g., ethyl cation, see above) are now believed to adopt non-classical structures. However, in many cases, the energy difference between the two possible "classical" structures and the "non-classical" one is very small, and it may be difficult to distinguish between the two possibilities experimentally.
Specific carbocations
Cyclopropylcarbinyl cations can be studied by NMR:[28][29]
In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups, indicating that the molecular conformation of this cation is not perpendicular (as in A), which possesses a mirror plane, but is bisected (as in B) with the empty p-orbital parallel to the cyclopropyl ring system:
In terms of bent bond theory, this preference is explained by assuming favorable orbital overlap between the filled cyclopropane bent bonds and the empty p-orbital.[30]
Pyramidal carbocation
| Pyramidal Carbocations | ||
|---|---|---|
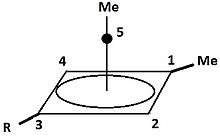 |
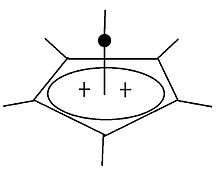 |
Besides the classical and non-classical a third class of carbonations can be distinguished: pyramidal carbocations. In these ions a single carbon atom hovers over a four- or five-sided polygon in effect forming a pyramid. The four-sided pyramidal ion will carry a charge of +1, the five sided pyramid will carry +2.
The crystal structure of [C6(CH3)6][SbF6]2•HSO3F confirms the pentagonal-pyramidal shape of the hexamethylbenzene dication.[31] |
| An example of the monovalent carbocation | An example of the divalent carbocation |
See also
- Armilenium
- Carbanion
- Carbene
- Carbo-mer
- Oxocarbenium
- Nonclassical Ion
References
- ↑ "Carbocation". Oxford Dictionaries. Oxford University Press. Retrieved 2016-01-21.
- ↑ Grützmacher, Hansjörg; Marchand, Christina M. (1997). "Heteroatom stabilized carbenium ions". Coord. Chem. Rev. 163: 287–344. doi:10.1016/S0010-8545(97)00043-X.
- ↑ Robert B. Grossman (2007-07-31). The Art of Writing Reasonable Organic Reaction Mechanisms. Springer Science & Business Media. pp. 105–. ISBN 978-0-387-95468-4.
- ↑ Olah, George A. (1972). "Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions". J. Am. Chem. Soc. 94 (3): 808–820. doi:10.1021/ja00758a020.
- ↑ McMurry, John. Organic chemistry (5th ed.). ISBN 0-534-37617-7.
- ↑ Yurkanis Bruice, Paula. Organic Chemistry (4th ed.). ISBN 0-13-140748-1.
- ↑ Clayden, Jonathan; Greeves, Nick; Warren, Stuart; Wothers, Peter (2001). Organic Chemistry (1st ed.). Oxford University Press. ISBN 978-0-19-850346-0.
- ↑ Fox, Marye Anne; Whitesell, James K. Organic Chemistry. ISBN 0-7637-0413-X.
- ↑ Merling, G. (1891). "Ueber Tropin". Berichte der deutschen chemischen Gesellschaft. 24 (2): 3108–3126. doi:10.1002/cber.189102402151. ISSN 0365-9496.
- ↑ Doering, W. von E.; Knox, L. H. (1954). "The Cycloheptatrienylium (Tropylium) Ion". J. Am. Chem. Soc. 76 (12): 3203–3206. doi:10.1021/ja01641a027.
- ↑ "On the Constitution of the Salts of Imido-Ethers and other Carbimide Derivatives". Am. Chem. J. 21: 101. ISSN 0096-4085.
- ↑ Meerwein, H.; Emster, K. van (1922). "About the equilibrium isomerism between bornyl chloride isobornyl chloride and camphene chlorohydrate". Berichte. 55: 2500.
- ↑ Rzepa, H. S.; Allan, C. S. M. (2010). "Racemization of Isobornyl Chloride via Carbocations: A Nonclassical Look at a Classic Mechanism". Journal of Chemical Education. 87 (2): 221. Bibcode:2010JChEd..87..221R. doi:10.1021/ed800058c.
- ↑ Doering, W. von E.; Saunders, M.; Boyton, H. G.; Earhart, H. W.; Wadley, E. F.; Edwards, W. R.; Laber, G. (1958). "The 1,1,2,3,4,5,6-heptamethylbenzenonium ion". Tetrahedron. 4 (1–2): 178–185. doi:10.1016/0040-4020(58)88016-3.
- ↑ Story, Paul R.; Saunders, Martin (1960). "The 7-norbornadienyl carbonium ion". J. Am. Chem. Soc. 82 (23): 6199. doi:10.1021/ja01508a058.
- ↑ Schleyer, Paul von R.; Watts, William E.; Fort, Raymond C.; Comisarow, Melvin B.; Olah, George A. (1964). "Stable Carbonium Ions. X.1 Direct Nuclear Magnetic Resonance Observation of the 2-Norbornyl Cation". J. Am. Chem. Soc. 86 (24): 5679–5680. doi:10.1021/ja01078a056.
- ↑ Saunders, Martin; Schleyer, Paul von R.; Olah, George A. (1964). "Stable Carbonium Ions. XI.1 The Rate of Hydride Shifts in the 2-Norbornyl Cation". J. Am. Chem. Soc. 86 (24): 5680–5681. doi:10.1021/ja01078a057.
- ↑ Anslyn, Eric V.; Dougherty, Dennis A. (2000). Modern Physical Organic Chemistry. Sausalito, CA: University Science Books. ISBN 1891389319.
- ↑ A., Carroll, Felix (2010). Perspectives on structure and mechanism in organic chemistry (2nd ed.). Hoboken, N.J.: John Wiley. ISBN 9780470276105. OCLC 286483846.
- ↑ 1937-, Carey, Francis A., (2007). Advanced organic chemistry. Sundberg, Richard J., 1938- (5th ed.). New York: Springer. ISBN 9780387448978. OCLC 154040953.
- ↑ Schultz, Jocelyn C.; Houle, F. A.; Beauchamp, J. L. (July 1984). "Photoelectron spectroscopy of 1-propyl, 1-butyl, isobutyl, neopentyl, and 2-butyl radicals: free radical precursors to high-energy carbonium ion isomers". Journal of the American Chemical Society. 106 (14): 3917–3927. doi:10.1021/ja00326a006. ISSN 0002-7863.
- ↑ Yamataka, Hiroshi; Ando, Takashi; Nagase, Shigeru; Hanamura, Mitsuyasu; Morokuma, Keiji (February 1984). "Ab initio MO calculations of isotope effects in model processes of neopentyl ester solvolysis". The Journal of Organic Chemistry. 49 (4): 631–635. doi:10.1021/jo00178a010. ISSN 0022-3263.
- ↑ Angelini, Giancarlo.; Hanack, Michael.; Vermehren, Jan.; Speranza, Maurizio. (1988-02-17). "Generation and trapping of an alkynyl cation". Journal of the American Chemical Society. 110 (4): 1298–1299. doi:10.1021/ja00212a052. ISSN 0002-7863.
- ↑ Olah, George A.; Prakash, G. K. Surya; Saunders, Martin (May 2002). "Conclusion of the classical-nonclassical ion controversy based on the structural study of the 2-norbornyl cation". Accounts of Chemical Research. 16 (12): 440–448. doi:10.1021/ar00096a003.
- ↑ George A. Olah - Nobel Lecture
- ↑ Yannoni, C. S.; Myhre, P. C.; Webb, Gretchen G. (November 1990). "Magic angle spinning nuclear magnetic resonance near liquid-helium temperatures. Variable-temperature CPMAS spectra of the 2-norbornyl cation to 6 K". Journal of the American Chemical Society. 112 (24): 8991–8992. doi:10.1021/ja00180a060. ISSN 0002-7863.
- ↑ Scholz, F.; Himmel, D.; Heinemann, F. W.; Schleyer, P. v R.; Meyer, K.; Krossing, I. (2013-07-05). "Crystal Structure Determination of the Nonclassical 2-Norbornyl Cation". Science. 341 (6141): 62–64. doi:10.1126/science.1238849. ISSN 0036-8075. PMID 23828938.
- ↑ Kabakoff, David S.; Namanworth, Eli (1970). "Nuclear magnetic double resonance studies of the dimethylcyclopropylcarbinyl cation. Measurement of the rotation barrier". J. Am. Chem. Soc. 92 (10): 3234–3235. doi:10.1021/ja00713a080.
- ↑ Pittman Jr., Charles U.; Olah, George A. (1965). "Stable Carbonium Ions. XVII.1a Cyclopropyl Carbonium Ions and Protonated Cyclopropyl Ketones". J. Am. Chem. Soc. 87 (22): 5123–5132. doi:10.1021/ja00950a026.
- ↑ Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A (2nd ed.).
- ↑ Malischewski, Moritz; Seppelt, K. (2016-11-25). "Crystal Structure Determination of the Pentagonal-Pyramidal Hexamethylbenzene Dication C6 (CH3 )6 2+". Angewandte Chemie International Edition. 56 (1): 368–370. doi:10.1002/anie.201608795. ISSN 1433-7851. line feed character in
|title=at position 90 (help)
External links

- Press Release The 1994 Nobel Prize in Chemistry". Nobelprize.org. 9 Jun 2010