Trichlorofluoromethane
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloro(fluoro)methane | |||
| Other names
Trichlorofluoromethane Fluorotrichloromethane Fluorochloroform Freon 11 CFC 11 R 11 Arcton 9 Freon 11A Freon 11B Freon HE Freon MF | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.812 | ||
| EC Number | 200-892-3 | ||
PubChem CID |
|||
| RTECS number | TB6125000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| CCl3F | |||
| Molar mass | 137.36 g·mol−1 | ||
| Appearance | Colorless liquid/gas | ||
| Odor | nearly odorless[1] | ||
| Density | 1.494 g/cm3 | ||
| Melting point | −110.48 °C (−166.86 °F; 162.67 K) | ||
| Boiling point | 23.77 °C (74.79 °F; 296.92 K) | ||
| 1.1 g/L (at 20 °C) | |||
| log P | 2.53 | ||
| Vapor pressure | 89 kPa at 20 °C 131 kPa at 30 °C | ||
| Hazards | |||
| Safety data sheet | See: data page ICSC 0047 | ||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published) |
26,200 ppm (rat, 4 hr) 100,000 ppm (rat, 20 min) 100,000 ppm (rat, 2 hr)[2] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
TWA 1000 ppm (5600 mg/m3)[1] | ||
REL (Recommended) |
C 1000 ppm (5600 mg/m3)[1] | ||
IDLH (Immediate danger) |
2000 ppm[1] | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon. It is a colorless, faint ethereal, and sweetish-smelling liquid that boils around room temperature.
Uses
It was the first widely used refrigerant. Because of its high boiling point (compared to most refrigerants), it can be used in systems with a low operating pressure, making the mechanical design of such systems less demanding than that of higher-pressure refrigerants R-12 or R-22.
R-11 is assigned an ozone depletion potential of 1.0, and U.S. production was ended on January 1, 1996.
Trichlorofluoromethane is used as a reference compound for fluorine-19 NMR studies.
Prior to the knowledge of the ozone depletion potential of chlorine in refrigerants and other possible harmful effects on the environment, trichlorofluoromethane was sometimes used as a cleaning/rinsing agent for low-pressure systems.[3]
Trichlorofluoromethane was formerly used in the drinking bird novelty, largely because it has a boiling point of 23.77℃ (74.79℉). The replacement, dichloromethane, boiling point 39.6℃ (103.3℉), requires a higher ambient temperature to work.
Moratorium
The substance is included in the production moratorium agreed in the Montreal Protocol of 1987 but in 2018 it emerged that in People's Republic of China it remains in widespread use as a blowing agent for polyurethane foam insulation in the construction industry and the hoped-for reduction in atmospheric concentration has not materialised.[4]
Gallery
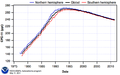 Hemispheric and Global mean concentrations of CFC-11 (NOAA/ESRL)
Hemispheric and Global mean concentrations of CFC-11 (NOAA/ESRL)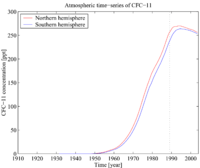 Time-series of atmospheric concentrations of CFC-11 (Walker et al., 2000)
Time-series of atmospheric concentrations of CFC-11 (Walker et al., 2000)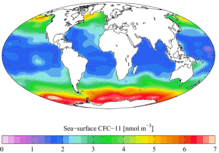 "Present day" (1990s) sea surface CFC-11 concentration
"Present day" (1990s) sea surface CFC-11 concentration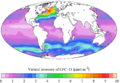 "Present day" (1990s) CFC-11 oceanic vertical inventory
"Present day" (1990s) CFC-11 oceanic vertical inventory
References
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0290". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Fluorotrichloromethane". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ "R-10 ,R-11 ,R-12 GASES - ملتقى التبريد والتكييف HVACafe". ملتقى التبريد والتكييف HVACafe (in Arabic). 2017-05-25. Retrieved 2018-05-18.
- ↑ McGrath, Matt (9 July 2018). "China 'home foam' gas key to ozone mystery". BBC News. Retrieved 9 July 2018.
External links
- CFC-11 NOAA/ESRL Global measurements
- Public health goal for trichlorofluoromethane in drinking water
- Names at webbook.nist.gov
- Data sheet at speclab.com
- International Chemical Safety Card 0047
- "NIOSH Pocket Guide to Chemical Hazards #0290". National Institute for Occupational Safety and Health (NIOSH).
- Phase change data at webbook.nist.gov
- Thermochemistry data at chemnet.ru
- ChemSub Online: Trichlorofluoromethane - CFC-11

