Integrin alpha L
Integrin, alpha L (antigen CD11A (p180), lymphocyte function-associated antigen 1; alpha polypeptide), also known as ITGAL, is a human gene which functions in the immune system. It is involved in cellular adhesion and costimulatory signaling. It is the target of the drug efalizumab.
Function
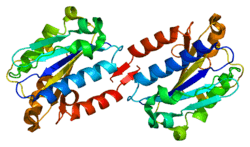
ITGAL encodes the integrin alpha L chain. Integrins are heterodimeric integral membrane proteins composed of an alpha chain and a beta chain. This I-domain containing alpha integrin combines with the beta 2 chain (ITGB2) to form the integrin lymphocyte function-associated antigen-1 (LFA-1), which is expressed on all leukocytes. LFA-1 plays a central role in leukocyte intercellular adhesion through interactions with its ligands, ICAMs 1-3 (intercellular adhesion molecules 1 through 3), and also functions in lymphocyte costimulatory signaling.[5]
CD11a is one of the two components, along with CD18, which form lymphocyte function-associated antigen-1.
Efalizumab acts as an immunosuppressant by binding to CD11a but was withdrawn in 2009 because it was associated with severe side effects.
Interactions
See also
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000005844 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000030830 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: ITGAL integrin, alpha L (antigen CD11A (p180), lymphocyte function-associated antigen 1; alpha polypeptide)".
- ↑ Lu C, Takagi J, Springer TA (May 2001). "Association of the membrane proximal regions of the alpha and beta subunit cytoplasmic domains constrains an integrin in the inactive state". J. Biol. Chem. 276 (18): 14642–8. doi:10.1074/jbc.M100600200. PMID 11279101.
- ↑ Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, Jun CD, McCormack A, Zhang R, Joachimiak A, Takagi J, Wang JH, Springer TA (Jan 2003). "Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation". Cell. 112 (1): 99–111. doi:10.1016/S0092-8674(02)01257-6. PMID 12526797.
- ↑ Yusuf-Makagiansar H, Makagiansar IT, Hu Y, Siahaan TJ (Dec 2001). "Synergistic inhibitory activity of alpha- and beta-LFA-1 peptides on LFA-1/ICAM-1 interaction". Peptides. 22 (12): 1955–62. doi:10.1016/S0196-9781(01)00546-0. PMID 11786177.
Further reading
- Lub M, van Kooyk Y, Figdor CG (1995). "Ins and outs of LFA-1". Immunol. Today. 16 (10): 479–83. doi:10.1016/0167-5699(95)80031-X. PMID 7576051.
- Dickeson SK, Santoro SA (1998). "Ligand recognition by the I domain-containing integrins". Cell. Mol. Life Sci. 54 (6): 556–66. doi:10.1007/s000180050184. PMID 9676575.
- Porter JC, Hogg N (1999). "Integrins take partners: cross-talk between integrins and other membrane receptors". Trends Cell Biol. 8 (10): 390–6. doi:10.1016/S0962-8924(98)01344-0. PMID 9789327.
- Giblin PA, Lemieux RM (2006). "LFA-1 as a key regulator of immune function: approaches toward the development of LFA-1-based therapeutics". Curr. Pharm. Des. 12 (22): 2771–95. doi:10.2174/138161206777947731. PMID 16918410.
- Maurer D, Holter W, Majdic O, Fischer GF, Knapp W (1991). "CD27 expression by a distinct subpopulation of human B lymphocytes". Eur. J. Immunol. 20 (12): 2679–84. doi:10.1002/eji.1830201223. PMID 1702722.
- Kalter DC, Gendelman HE, Meltzer MS (1992). "Inhibition of human immunodeficiency virus infection in monocytes by monoclonal antibodies against leukocyte adhesion molecules". Immunol. Lett. 30 (2): 219–27. doi:10.1016/0165-2478(91)90029-A. PMID 1757107.
- Alvarez V, Pulido R, Campanero MR, Paraiso V, de Landázuri MO, Sánchez-Madrid F (1992). "Differentially regulated cell surface expression of leukocyte adhesion receptors on neutrophils". Kidney Int. 40 (5): 899–905. doi:10.1038/ki.1991.291. PMID 1762294.
- Valentin A, Lundin K, Patarroyo M, Asjö B (1990). "The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells". J. Immunol. 144 (3): 934–7. PMID 1967280.
- Larson RS, Corbi AL, Berman L, Springer T (1989). "Primary structure of the leukocyte function-associated molecule-1 alpha subunit: an integrin with an embedded domain defining a protein superfamily". J. Cell Biol. 108 (2): 703–12. doi:10.1083/jcb.108.2.703. PMC 2115430. PMID 2537322.
- Hildreth JE, Orentas RJ (1989). "Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation". Science. 244 (4908): 1075–8. doi:10.1126/science.2543075. PMID 2543075.
- Sanders ME, Makgoba MW, Sharrow SO, Stephany D, Springer TA, Young HA, Shaw S (1988). "Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production". J. Immunol. 140 (5): 1401–7. PMID 2894392.
- Corbi AL, Larson RS, Kishimoto TK, Springer TA, Morton CC (1988). "Chromosomal location of the genes encoding the leukocyte adhesion receptors LFA-1, Mac-1 and p150,95. Identification of a gene cluster involved in cell adhesion". J. Exp. Med. 167 (5): 1597–607. doi:10.1084/jem.167.5.1597. PMC 2188934. PMID 3284962.
- te Velde AA, Keizer GD, Figdor CG (1987). "Differential function of LFA-1 family molecules (CD11 and CD18) in adhesion of human monocytes to melanoma and endothelial cells". Immunology. 61 (3): 261–7. PMC 1453405. PMID 3301632.
- Qu A, Leahy DJ (1995). "Crystal structure of the I-domain from the CD11a/CD18 (LFA-1, alpha L beta 2) integrin". Proc. Natl. Acad. Sci. U.S.A. 92 (22): 10277–81. doi:10.1073/pnas.92.22.10277. PMC 40779. PMID 7479767.
- Turner ML, McIlwaine K, Anthony RS, Parker AC (1995). "Differential expression of cell adhesion molecules by human hematopoietic progenitor cells from bone marrow and mobilized adult peripheral blood". Stem Cells. 13 (3): 311–6. doi:10.1002/stem.5530130312. PMID 7542116.
- Marzusch K, Ruck P, Geiselhart A, Handgretinger R, Dietl JA, Kaiserling E, Horny HP, Vince G, Redman CW (1993). "Distribution of cell adhesion molecules on CD56++, CD3-, CD16- large granular lymphocytes and endothelial cells in first-trimester human decidua". Hum. Reprod. 8 (8): 1203–8. PMID 7691868.
- Capobianchi MR, Ameglio F, Cordiali Fei P, Castilletti C, Mercuri F, Fais S, Dianzani F (1994). "Coordinate induction of interferon alpha and gamma by recombinant HIV-1 glycoprotein 120". AIDS Res. Hum. Retroviruses. 9 (10): 957–62. doi:10.1089/aid.1993.9.957. PMID 7904170.
- Berman PW, Nakamura GR (1994). "Adhesion mediated by intercellular adhesion molecule 1 attenuates the potency of antibodies that block HIV-1 gp160-dependent syncytium formation". AIDS Res. Hum. Retroviruses. 10 (5): 585–93. doi:10.1089/aid.1994.10.585. PMID 7917519.
- Chirmule N, Oyaizu N, Saxinger C, Pahwa S (1994). "Nef protein of HIV-1 has B-cell stimulatory activity". AIDS. 8 (6): 733–4. doi:10.1097/00002030-199406000-00002. PMID 8086129.
External links
- CD11a+Antigen at the US National Library of Medicine Medical Subject Headings (MeSH)
- ITGAL Info with links in the Cell Migration Gateway
- Human ITGAL genome location and ITGAL gene details page in the UCSC Genome Browser.