Eucalyptol
Eucalyptol is a natural organic compound that is a colorless liquid. It is a cyclic ether and a monoterpenoid.
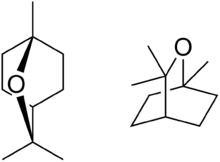 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octane | |||
| Other names
1,8-Cineole 1,8-Epoxy-p-menthane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.006.757 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C10H18O | |||
| Molar mass | 154.249 g/mol | ||
| Density | 0.9225 g/cm3 | ||
| Melting point | 2.9 °C (37.2 °F; 276.0 K) | ||
| Boiling point | 176–177 °C (349–351 °F; 449–450 K) | ||
| −116.3×10−6 cm3/mol | |||
| Pharmacology | |||
| R05CA13 (WHO) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Eucalyptol is also known by a variety of synonyms: 1,8-cineol, 1,8-cineole, cajeputol, 1,8-epoxy-p-menthane, 1,8-oxido-p-menthane, eucalyptol, eucalyptole, 1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane, cineol, and cineole.
In 1870, F. S. Cloez identified and ascribed the name "eucalyptol" to the dominant portion of Eucalyptus globulus oil.[1]
Composition
The content of eucalyptol is 90% of eucalyptus oil,[1] leading to its name. Wormwood, rosemary, common sage, Cannabis sativa, and other aromatic plant foliage contain eucalyptol. Eucalyptol may be toxic if ingested.[2]
Properties
Eucalyptol has a fresh mint-like smell and a spicy, cooling taste. It is insoluble in water, but miscible with ether, ethanol, and chloroform. The boiling point is 176°C and the flash point is 49°C. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.
Uses
Flavoring and fragrance
Because of its pleasant, spicy aroma and taste, eucalyptol is used in flavorings, fragrances, and cosmetics. Cineole-based eucalyptus oil is used as a flavouring at low levels (0.002%) in various products, including baked goods, confectionery, meat products, and beverages.[3] In a 1994 report released by five top cigarette companies, eucalyptol was listed as one of the 599 additives to cigarettes.[4] It is claimed to be added to improve the flavor.
Eucalyptol is an ingredient in commercial mouthwashes, and has been used in traditional medicine as a cough suppressant.[5]
Insecticide and repellent
Eucalyptol is used as an insecticide and insect repellent.[6][7]
In contrast, eucalyptol is one of many compounds that are attractive to males of various species of orchid bees, which gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.[8] One such study with Euglossa imperialis, a nonsocial orchid bee species, has shown that the presence of cineole (also eucalyptol) elevates territorial behavior and specifically attracts the male bees. It was even observed that these males would periodically leave their territories to forage for chemicals such as cineole, thought to be important for attracting and mating with females, to synthesize pheromones.[9]
Toxicology
In higher-than-normal doses, eucalyptol is hazardous via ingestion, skin contact, or inhalation. It can have acute health effects on behavior, the respiratory tract, and the nervous system. The acute oral LD50 is 2480 mg/kg (rat). It is classified as a reproductive toxin for females and a suspected reproductive toxin for males.[2]
Pharmacodynamics
It is known that eucalyptol has anti-inflammatory properties and researchers have suggested these, and other effects such as soothing sensations, may be mediated by the ion channel TRPM8.[10] The same channel is also activated by menthol.
List of plants that contain the chemical
- Artemisia tridentata[11]
- Cannabis[12]
- Cinnamomum camphora, camphor laurel (50%)[13]
- Eucalyptus cneorifolia
- Eucalyptus dives
- Eucalyptus dumosa
- Eucalyptus globulus[14]
- Eucalyptus goniocalyx
- Eucalyptus horistes
- Eucalyptus kochii
- Eucalyptus leucoxylon
- Eucalyptus oleosa
- Eucalyptus polybractea
- Eucalyptus radiata
- Eucalyptus rossii
- Eucalyptus sideroxylon
- Eucalyptus smithii
- Eucalyptus staigeriana[15]
- Eucalyptus tereticornis
- Eucalyptus viridis
- Hedychium coronarium, butterfly lily[16][17][18][19]
- Helichrysum gymnocephalum[20]
- Kaempferia galanga, galangal, (5.7%)[21]
- Laurus nobilis, bay laurel, (45%)
- Melaleuca alternifolia, tea tree, (0–15%)
- Salvia lavandulifolia, Spanish sage (13%)[22]
- Turnera diffusa, damiana[23]
- Umbellularia californica, pepperwood (22.0%)[24]
- Zingiber officinale, ginger
Compendial status
- British Pharmacopoeia[25]
- Martindale: The Extra Pharmacopoeia 31[26]
N.B. Listed as "cineole" in some pharmacopoeias.
References
- Boland, D. J.; Brophy, J. J.; House, A. P. N. (1991). Eucalyptus Leaf Oils: Use, Chemistry, Distillation and Marketing. Melbourne: Inkata Press. p. 6. ISBN 0-909605-69-6.
- "Material Safety Data Sheet – Cineole". ScienceLab. Archived from the original on 29 August 2012. Retrieved 27 September 2012.
- Harborne, J. B.; Baxter, H. (30 August 2001). Chemical Dictionary of Economic Plants. ISBN 0-471-49226-4.
- "Cigarette Ingredients – Chemicals in Cigarettes". New York State Department of Health. Retrieved 28 July 2014.
- "Tea tree oil". Drugs.com. 17 June 2019. Retrieved 31 July 2019.
- Klocke, J. A.; Darlington, M. V.; Balandrin, M. F. (December 1987). "8-Cineole (Eucalyptol), a Mosquito Feeding and Ovipositional Repellent from Volatile Oil of Hemizonia fitchii (Asteraceae)". Journal of Chemical Ecology. 13 (12): 2131–41. doi:10.1007/BF01012562. PMID 24301652.
- Sfara, V.; Zerba, E. N.; Alzogaray, R. A. (May 2009). "Fumigant Insecticidal Activity and Repellent Effect of Five Essential Oils and Seven Monoterpenes on First-Instar Nymphs of Rhodnius prolixus". Journal of Medical Entomology. 46 (3): 511–515. doi:10.1603/033.046.0315. PMID 19496421.
- Schiestl, F. P.; Roubik, D. W. (2004). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. PMID 12647866.
- Schemske, Douglas W.; Lande, Russell (1984). "Fragrance collection and territorial display by male orchid bees". Animal Behaviour. 32 (3): 935–937. doi:10.1016/s0003-3472(84)80184-0.
- Caceres, Ana I.; Liu, Boyi; Jabba, Sairam V.; Achanta, Satyanarayana; Morris, John B.; Jordt, Sven-Eric (May 2017). "Transient Receptor Potential Cation Channel Subfamily M Member 8 channels mediate the anti-inflammatory effects of eucalyptol". British Journal of Pharmacology. 174 (9): 867–879. doi:10.1111/bph.13760. ISSN 1476-5381. PMC 5387001. PMID 28240768.
- Crowell, M.M.; et al. (2018). "Dietary partitioning of toxic leaves and fibrous stems differs between sympatric specialist and generalist mammalian herbivores". Journal of Mammalogy. 99 (3): 565–577. doi:10.1093/jmammal/gyy018.
- McPartland, J. M.; Russo, E. B. (2001). "Cannabis and cannabis extracts: greater than the sum of their parts?". Journal of Cannabis Therapeutics. 1 (3–4): 103–132. doi:10.1300/J175v01n03_08. Retrieved 20 September 2013.
- Stubbs, B. J.; Brushett, D. (2001). "Leaf oil of Cinnamomum camphora (L.) Nees and Eberm. From Eastern Australia". Journal of Essential Oil Research. 13 (1): 51–54. doi:10.1080/10412905.2001.9699604.
- Maciel, M. V.; Morais, S. M.; Bevilaqua, C. M.; Silva, R. A.; Barros, R. S.; Sousa, R. N.; Sousa, L. C.; Brito, E. S.; Souza Neto, M. A. (2010). "Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis" (PDF). Veterinary Parasitology. 167 (1): 1–7. doi:10.1016/j.vetpar.2009.09.053. PMID 19896276.
- Gilles, M.; Zhao, J.; An, M.; Agboola, S. (2010). "Chemical Composition and Antimicrobial Properties of Essential Oils of three Australian Eucalyptus Species". Food Chemistry. 119 (2): 731–737. doi:10.1016/j.foodchem.2009.07.021.
- Ali, S.; Sotheeswaran, S.; Tuiwawa, M.; Smith, R. (2002). "Comparison of the Composition of the Essential Oils of Alpinia and Hedychium Species—Essential Oils of Fijian Plants, Part 1". Journal of Essential Oil Research. 14 (6): 409–411. doi:10.1080/10412905.2002.9699904.
- Joy, B.; Rajan, A.; Abraham, E. (2007). "Antimicrobial Activity and Chemical Composition of Essential Oil from Hedychium coronarium". Phytotherapy Research. 21 (5): 439–443. doi:10.1002/ptr.2091. PMID 17245683.
- Martins, M.; Caravante, A.; Appezzato-Da-Glória, B.; Soares, M.; Moreira, R.; Santos, L. (2010). "Anatomical characterization and phytochemistry of leaves and rhizomes of Hedychium coronarium J. König (Zingiberaceae)". Rev. Bras. Plantas Med. 12 (2): 179–187. doi:10.1590/s1516-05722010000200009.
- Santos, S. B.; Pedralli, G.; Meyer, S. T. (2005). "Aspectos da fenologia e ecologia de Hedychium coronarium (Zingiberaceae) na estação ecológica do Tripuí, Ouro Preto MG". Planta Daninha (in Portuguese). 23 (2): 175–180. doi:10.1590/s0100-83582005000200002.
- Möllenbeck, S.; König, T.; Schreier, P.; Schwab, W.; Rajaonarivony, J.; Ranarivelo, L. (1997). "Chemical Composition and Analyses of Enantiomers of Essential Oils from Madagascar". Flavour and Fragrance Journal. 12 (2): 63. doi:10.1002/(SICI)1099-1026(199703)12:2<63::AID-FFJ614>3.0.CO;2-Z.
- Wong, K. C.; Ong, K. S.; Lim, C. L. (2006). "Composition of the Essential Oil of Rhizomes of Kaempferia galanga L.". Flavour and Fragrance Journal. 7 (5): 263–266. doi:10.1002/ffj.2730070506.
- Perry, N. S.; Houghton, P. J.; Theobald, A.; Jenner, P.; Perry, E. K. (2000). "In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes". J Pharm Pharmacol. 52 (7): 895–902. doi:10.1211/0022357001774598. PMID 10933142.
- Balch, P. A. (2002). Prescription for Nutritional Healing: the A to Z Guide to Supplements. Penguin. p. 233. ISBN 978-1-58333-143-9.
- Kelsey, R. G.; McCuistion, O.; Karchesy, J. (2007). "Bark and Leaf Essential Oil of Umbellularia californica, California Bay Laurel, from Oregon". Natural Product Communications. 2 (7): 779–780. doi:10.1177/1934578X0700200715.
- The British Pharmacopoeia Secretariat (2009). "Index, BP 2009" (PDF). Archived from the original (PDF) on 11 April 2009. Retrieved 5 July 2009.
- Therapeutic Goods Administration. "Chemical Substances" (PDF). tga.gov.au. Archived from the original (PDF) on 2 July 2009. Retrieved 5 July 2009.
Further reading
- Boland, D. J.; Brophy, J. J.; House, A. P. N. (1991). Eucalyptus Leaf Oils: Use, Chemistry, Distillation and Marketing. Melbourne: Inkata Press. ISBN 0-909605-69-6.
External links
- "Eucalyptus". Botanical.com.
- "Oleum Eucalypti, B.P. Oil of Eucalyptus". Henriette's Herbal.
- "MSDS – Safety data for eucalyptol". Oxford University Chemistry Department. Archived from the original on 11 October 2007. Retrieved 7 January 2008.