Mavatrep
Mavatrep (JNJ‐39439335) is a TRPV1 receptor selective competitive antagonist.[1] It is an investigational analgesic that may be a potential treatment for analgesia and/or inflammation.
 | |
| Clinical data | |
|---|---|
| Other names | JNJ-39439335 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
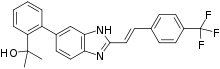
| Formula | C25H21F3N2O |
| Molar mass | 422.451 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Phase I trials have been completed in healthy Japanese and Caucasian volunteers.[1][2]
Potential common adverse effects include thermohypoesthesia, chills, feeling cold, and feeling hot.[2]
Pharmacokinetics
When administered orally once a day, mavatrep reached steady-state in healthy volunteers in approximately 14 days.[2] It has a relatively long half life between 68–101 hours in Japanese subjects and between 82–130 hours in Caucasian subjects.[2]
Mavatrep is largely eliminated nonrenally. Mavatrep appears to be metabolized into two primary metabolites which are also eliminated nonrenally.[2]
References
- Manitpisitkul, Prasarn; Shalayda, Kevin; Russell, Lucille; Sanga, Panna; Williams, Yinka; Solanki, Bhavna; Caruso, Joseph; Moyer, John A. (2018). "Bioavailability and Pharmacokinetics of TRPV1 Antagonist Mavatrep (JNJ-39439335) Tablet and Capsule Formulations in Healthy Men: Two Open-Label, Crossover, Single-Dose Phase 1 Studies". Clinical Pharmacology in Drug Development. 7 (7): 699–711. doi:10.1002/cpdd.412. ISSN 2160-7648. PMID 29125700.
- Manitpisitkul, Prasarn; Shalayda, Kevin; Russell, Lucille; Sanga, Panna; Solanki, Bhavna; Caruso, Joseph; Iwaki, Yuki; Moyer, John A. (2018). "Pharmacokinetics and Safety of Mavatrep (JNJ-39439335), a TRPV1 Antagonist in Healthy Japanese and Caucasian Men: A Double-Blind, Randomized, Placebo-Controlled, Sequential-Group Phase 1 Study". Clinical Pharmacology in Drug Development. 7 (7): 712–726. doi:10.1002/cpdd.413. ISSN 2160-7648. PMID 29125703.