Allyl isothiocyanate
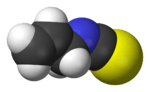
Allyl isothiocyanate (AITC) is the organosulfur compound with the formula CH2CHCH2NCS. This colorless oil is responsible for the pungent taste of mustard, radish, horseradish, and wasabi. This pungency and the lachrymatory effect of AITC are mediated through the TRPA1 and TRPV1 ion channels.[1][2][3] It is slightly soluble in water, but more soluble in most organic solvents.[4]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Isothiocyanatoprop-1-ene | |
| Other names
Synthetic mustard oil | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.281 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1545 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H5NS | |
| Molar mass | 99.15 g·mol−1 |
| Density | 1.013–1.020 g/cm3 |
| Melting point | −102 °C (−152 °F; 171 K) |
| Boiling point | 148 to 154 °C (298 to 309 °F; 421 to 427 K) |
| Hazards | |
| GHS pictograms |    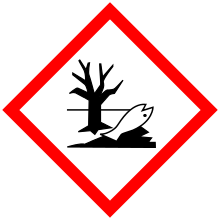 |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H301, H310, H315, H319, H330, H335, H400, H410 |
| P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P270, P271, P273, P280, P284, P301+310, P302+350, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P320, P321 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis and biological functions
Allyl isothiocyanate can be obtained from the seeds of black mustard (Brassica nigra) or brown Indian mustard (Brassica juncea). When these mustard seeds are broken, the enzyme myrosinase is released and acts on a glucosinolate known as sinigrin to give allyl isothiocyanate.
Allyl isothiocyanate serves the plant as a defense against herbivores; since it is harmful to the plant itself , it is stored in the harmless form of the glucosinolate, separate from the myrosinase enzyme. When an animal chews the plant, the allyl isothiocyanate is released, repelling the animal. This chemical has recently been shown to strongly repel fire ants, (Solenopsis invicta)[5]
Commercial and other applications
Allyl isothiocyanate is produced commercially by the reaction of allyl chloride and potassium thiocyanate:[4]
- CH2=CHCH2Cl + KSCN → CH2=CHCH2NCS + KCl
The product obtained in this fashion is sometimes known as synthetic mustard oil. Allyl isothiocyanate can also be liberated by dry distillation of the seeds. The product obtained in this fashion is known as volatile oil of mustard and is usually around 92% pure. It is used principally as a flavoring agent in foods. Synthetic allyl isothiocyanate is used as an insecticide, as an anti-mold agent [6] bacteriocide,[7] and nematocide, and is used in certain cases for crop protection.[4]
Hydrolysis of allyl isothiocyanate gives allylamine.[8]
Safety
Allyl isothiocyanate has an LD50 of 151 mg/kg and is a lachrymator (similar to tear gas or mace).[4]
Oncology
Based on in vitro experiments and animal models, allyl isothiocyanate exhibits many of the desirable attributes of a cancer chemopreventive agent.[9]
See also
- Mustard plaster, traditional home remedy
- Piperine, the active piquant chemical in black pepper
- Capsaicin, the active piquant chemical in chili peppers
- Allicin, the active piquant flavor chemical in raw garlic
References
- Everaerts, W.; Gees, M.; Alpizar, Y. A.; Farre, R.; Leten, C.; Apetrei, A.; Dewachter, I.; van Leuven, F.; et al. (2011). "The Capsaicin Receptor TRPV1 is a Crucial Mediator of the Noxious Effects of Mustard Oil". Current Biology. 21 (4): 316–321. doi:10.1016/j.cub.2011.01.031. PMID 21315593.
- Brône, B.; Peeters, P. J.; Marrannes, R.; Mercken, M.; Nuydens, R.; Meert, T.; Gijsen, H. J. (2008). "Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor". Toxicology and Applied Pharmacology. 231 (2): 150–156. doi:10.1016/j.taap.2008.04.005. PMID 18501939.
- Ryckmans, T.; Aubdool, A. A.; Bodkin, J. V.; Cox, P.; Brain, S. D.; Dupont, T.; Fairman, E.; Hashizume, Y.; Ishii, N.; et al. (2011). "Design and Pharmacological Evaluation of PF-4840154, a Non-Electrophilic Reference Agonist of the TrpA1 Channel". Bioorganic & Medicinal Chemistry Letters. 21 (16): 4857–4859. doi:10.1016/j.bmcl.2011.06.035. PMID 21741838.
- Romanowski, F.; Klenk, H. "Thiocyanates and Isothiocyanates, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_749.
- Hashimoto, Y., Yoshimura, M. & Huang, RN. Appl Entomol Zool (2019). https://doi.org/10.1007/s13355-019-00613-5
- US Patent No. 8691042
- Shin, I. S.; Masuda, H.; Naohide, K. (2004). "Bactericidal Activity of Wasabi (Wasabia japonica) against Helicobacter pylori". International Journal of Food Microbiology. 94 (3): 255–261. doi:10.1016/S0168-1605(03)00297-6. PMID 15246236.
- Leffler, M. T. (1938). "Allylamine". Organic Syntheses. 18: 5.; Collective Volume, 2, p. 24
- Zhang, Y (2010). "Allyl isothiocyanate as a cancer chemopreventive phytochemical". Molecular Nutrition & Food Research. 54 (1): 127–35. doi:10.1002/mnfr.200900323. PMC 2814364. PMID 19960458.