Nonivamide
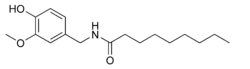
Nonivamide, also called pelargonic acid vanillylamide or PAVA, is an organic compound and a capsaicinoid. It is an amide of pelargonic acid (n-nonanoic acid) and vanillyl amine. It is present in chili peppers,[2] but is commonly manufactured synthetically. It is more heat-stable than capsaicin.
 | |
| Names | |
|---|---|
| IUPAC name
N-[(4-Hydroxy-3-methoxyphenyl)methyl]nonanamide | |
| Other names
Pseudocapsaicin; Vanillyl-N-nonylamide; Vanillylamide of n-nonanoic acid; VNA; Nonylic acid vanillyl amide; Pelargonic acid vanillylamide (PAVA); Pelargonyl vanillyl amide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.713 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H27NO3 | |
| Molar mass | 293.407 g·mol−1 |
| Appearance | White to off-white powder |
| Odor | Pungent |
| Density | 1.10 g/cm3 |
| Melting point | 54 °C (129 °F; 327 K) |
| Insoluble | |
| Solubility | Soluble in methanol |
| Hazards | |
| Flash point | 190 °C (374 °F; 463 K) (closed cup) |
| 330 °C (626 °F; 603 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
511 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Nonivamide | |
|---|---|
| Heat | Above peak |
| Scoville scale | 9,200,000[1] SHU |
Nonivamide is used as a food additive to add pungency to seasonings, flavorings, and spice blends. It is also used in the confectionery industry to create a hot sensation, and in the pharmaceutical industry in some formulations as a cheaper alternative to capsaicin.
Like capsaicin, it can deter mammals (but not birds or insects) from consuming plants or seeds (e.g. squirrels and bird feeder seeds).[3] This is consistent with nonivamide's role as a TRPV1 ion channel agonist. Mammalian TRPV1 is activated by heat and capsaicin, but the avian form is insensitive to capsaicin.[4]
Nonivamide is used (under the name PAVA) as the payload in "less-lethal munitions" such as the Fabrique Nationale Herstal FN 303[5] or as the active ingredient in most pepper sprays[3] – in both applications, the idea is to temporarily incapacitate people so that they can either be detained prior to arrest or deterred from acts of violence toward law-enforcement personnel or third parties (such as rioting or other group violence).
Treatment
There are various treatments to combat the effects of nonivamide. One popular method includes administering a one-to-one solution of Milk of Magnesia and water to the eyes. Doctors also recommend not using oils or creams on the skin and to not wear contacts if one expects to encounter nonivamide. [6]
See also
References
- Govindarajan, Sathyanarayana (1991). "Capsicum — Production, Technology, Chemistry, and Quality. Part V. Impact on Physiology, Pharmacology, Nutrition, and Metabolism; Structure, Pungency, Pain, and Desensitization Sequences". Critical Reviews in Food Science and Nutrition. 29 (6): 435–474. doi:10.1080/10408399109527536. PMID 2039598.
- Howard L. Constant, Geoffrey A. Cordell and Dennis P. West (1996). "Nonivamide, a Constituent of Capsicum oleoresin". J. Nat. Prod. 59 (4): 425–426. doi:10.1021/np9600816.
- http://www.aversiontech.com/hot-and-spicy/nonivamide-pava/Retrieved 16 July 2010 Archived 31 December 2015 at the Wayback Machine
- Rohm, Barbara; Riedel, Annett; Ley, Jakob P; Widder, Sabine; Krammer, Gerhard E; Somoza, Veronika (2015). "Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme a synthetase activity in Caco-2 cells". Food & Function. 6: 172. doi:10.1039/C4FO00435C.
- "The FN 303 Less Lethal Launcher". Archived from the original on 2013-05-04. Retrieved 2013-04-14.
- Brown, Dr. Ernest (2020-05-31). "Bike-riding doctor helps protesters recover from pepper spray attacks". WUSA9. Retrieved 2020-06-01.