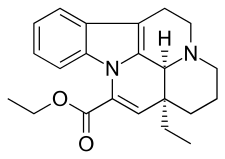
Vinpocetine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 56.6 +/- 8.9% |
| Metabolism | hepatic |
| Elimination half-life | 2.54 +/- 0.48 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.050.917 |
| Chemical and physical data | |
| Formula | C22H26N2O2 |
| Molar mass | 350.454 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Vinpocetine (brand names: Cavinton, Intelectol; chemical name: ethyl apovincaminate) is a synthetic derivative of the vinca alkaloid vincamine (sometimes described as "a synthetic ethyl ester of apovincamine"),[1] an extract from the lesser periwinkle plant. Vinpocetine was first isolated from the plant in 1975 by the Hungarian chemist Csaba Szántay. The mass production of the synthetic compound was started in 1978 by the Hungarian pharmaceutical company Richter Gedeon.
Vinpocetine is not FDA approved in the United States for therapeutic use. The U.S. Food & Drug Administration (FDA) has ruled that vinpocetine, due to its synthetic nature and proposed therapeutic uses, was ineligible to be marketed as dietary supplement under the Federal Food, Drug, and Cosmetic Act (FDCA).[2][3][4][5]
Controlled clinical trials
As of 2003 only three controlled clinical trials had tested "older adults with memory problems".[6] However, a 2003 Cochrane review determined that the results were inconclusive.[7]
In vitro and animal studies
Kindling models in rats has shown vinpocetine to exhibit anticonvulsant properties. The most pronounced anticonvulsant effects were observed in Pentylenetetrazole (PTZ)-kindled rats although there was also an effect on amygdala-kindled and neocortically-kindled rats.[8] Vinpocetine has also been shown to abolish [3H]Glu release after in vivo exposure to 4-aminopyridine (4-AP) which suggests an important mechanism for vinpocetine anticonvulsant activity.[9]
Vinpocetine has been investigated in animal models as a potential anti-inflammatory agent.[10] [11] Vinpocetine inhibits the up-regulation of NF-κB by TNFα in various cell tests. Reverse transcription polymerase chain reaction also shows that it reduced the TNFα-induced expression of the mRNA of proinflammatory molecules such as interleukin-1 beta, monocyte chemoattractant protein-1 (MCP-1), and vascular cell adhesion molecule-1 (VCAM-1). In mice, vinpocetine reduced lipopolysaccharide inoculation induced polymorphonuclear neutrophil infiltration into the lung.[10][11]
Mechanism of action
Vinpocetine acts as a phosphodiesterase (PDE) type-1 inhibitor in isolated rabbit aorta,[12] Independent of vinpocetine's action on PDE, vinpocetine inhibits IKK preventing IκB degradation and the following translocation of NF-κB to the cell nucleus.[10][11]
Increases in neuronal levels of DOPAC, a metabolic breakdown product of dopamine, have been shown to occur in striatal isolated nerve endings as a result of exposure to vinpocetine.[13] Such an effect is consistent with the biogenic pharmacology of reserpine, a structural relative of vinpocetine.[13] However, this effect tends to be reversible upon cessation of vinpocetine administration, with full remission typically occurring within 3–4 weeks.
Vinpocetine has been reported to have cerebral blood-flow enhancing[14] and neuroprotective effects.[15]
Side effects
Some studies have noted flushing, rashes, or minor gastrointestinal problems in some subjects; however, these side effects did not warrant discontinuation of the medication.[16] Some users have reported headaches, especially at doses above 15 milligrams per day, as well as occasional upset stomach. The safety of vinpocetine in pregnant women has not been evaluated. Vinpocetine has been implicated in one case to induce agranulocytosis,[17] a serious condition in which granulocytes are markedly decreased. Some people have anecdotally noted that their continued use of vinpocetine reduces immune function. Commission E warned that vinpocetine reduced immune function could cause apoptosis (cellular death) in the long term.[18]
References
- ↑ Lörincz C, Szász K, Kisfaludy L (1976). "The synthesis of ethyl apovincaminate". Arzneimittel-Forschung. 26 (10a): 1907. PMID 1037211.
- ↑ Schmitt, Rick (January 12, 2017). "Marketers exploit the aged with unproven brain-health claims". Newsweek. Retrieved January 18, 2016.
- ↑ Hank, Schulz. "eligible to be marketed as dietary supplement under the Federal Food, Drug, and Cosmetic Act (FDCA)". NutraIngredients. William Reed Business Media, England. Retrieved September 8, 2016.
- ↑ "FDA Concludes Vinpocetine Ineligible as a Dietary Ingredient". Nutraceuticals World. Rodman Media. September 20, 2016. Retrieved January 18, 2017.
- ↑ Schmitt, Rick (February 3, 2017). "Dubious doses". Newsweek. Retrieved September 24, 2017.
- ↑ McDaniel MA, Maier SF, Einstein GO (2003). ""Brain-specific" nutrients: a memory cure?". Nutrition. 19 (11–12): 957–75. doi:10.1016/S0899-9007(03)00024-8. PMID 14624946.
- ↑ Szatmari SZ, Whitehouse PJ (2003). Szatmári S, ed. "Vinpocetine for cognitive impairment and dementia". The Cochrane Database of Systematic Reviews (1): CD003119. doi:10.1002/14651858.CD003119. PMID 12535455.
- ↑ Schmidt J (1990). "Comparative studies on the anticonvulsant effectiveness of nootropic drugs in kindled rats". Biomedica Biochimica Acta. 49 (5): 413–9. PMID 2271012.
- ↑ Sitges M, Sanchez-Tafolla BM, Chiu LM, Aldana BI, Guarneros A (October 2011). "Vinpocetine inhibits glutamate release induced by the convulsive agent 4-aminopyridine more potently than several antiepileptic drugs". Epilepsy Research. 96 (3): 257–66. doi:10.1016/j.eplepsyres.2011.06.006. PMID 21737246.
- 1 2 3 Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Li JD, Yan C (May 2010). "Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism". Proceedings of the National Academy of Sciences of the United States of America. 107 (21): 9795–800. Bibcode:2010PNAS..107.9795J. doi:10.1073/pnas.0914414107. PMC 2906898. PMID 20448200.
- 1 2 3 Medina AE (June 2010). "Vinpocetine as a potent antiinflammatory agent". Proceedings of the National Academy of Sciences of the United States of America. 107 (22): 9921–2. Bibcode:2010PNAS..107.9921M. doi:10.1073/pnas.1005138107. PMC 2890434. PMID 20495091.
- ↑ Hagiwara M, Endo T, Hidaka H (February 1984). "Effects of vinpocetine on cyclic nucleotide metabolism in vascular smooth muscle". Biochemical Pharmacology. 33 (3): 453–7. doi:10.1016/0006-2952(84)90240-5. PMID 6322804.
- 1 2 Trejo F, Nekrassov V, Sitges M (August 2001). "Characterization of vinpocetine effects on DA and DOPAC release in striatal isolated nerve endings". Brain Research. 909 (1–2): 59–67. doi:10.1016/S0006-8993(01)02621-X. PMID 11478921.
- ↑ Szilágyi G, Nagy Z, Balkay L, Boros I, Emri M, Lehel S, Márián T, Molnár T, Szakáll S, Trón L, Bereczki D, Csiba L, Fekete I, Kerényi L, Galuska L, Varga J, Bönöczk P, Vas A, Gulyás B (March 2005). "Effects of vinpocetine on the redistribution of cerebral blood flow and glucose metabolism in chronic ischemic stroke patients: a PET study". Journal of the Neurological Sciences. 229-230: 275–84. doi:10.1016/j.jns.2004.11.053. PMID 15760651.
- ↑ Dézsi L, Kis-Varga I, Nagy J, Komlódi Z, Kárpáti E (2002). "[Neuroprotective effects of vinpocetine in vivo and in vitro. Apovincaminic acid derivatives as potential therapeutic tools in ischemic stroke]". Acta Pharmaceutica Hungarica. 72 (2): 84–91. PMID 12498034.
- ↑ "Vinpocetine. Monograph" (PDF). Alternative Medicine Review. 7 (3): 240–3. June 2002. PMID 12126465.
- ↑ Shimizu Y, Saitoh K, Nakayama M, Suto K, Raikohara R, Nemoto T. "Agranulocytosis induced by vinpocetine". Medicine Online. Retrieved 8 March 2008.
- ↑ Blumenthal M, Busse WR, Bundesinstitut für Arzneimittel und Medizinprodukte (Germany) (1999). The Complete German Commission E monographs : therapeutic guide to herbal medicines (Reprint ed.). Austin, Tex.: American Botanical Council. ISBN 978-0-9655555-0-0.