Crisaborole
 | |
| Clinical data | |
|---|---|
| Trade names | Eucrisa, /juːˈkrɪsə/ yoo-KRIS-ə |
| Routes of administration | Topical (ointment) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C14H10BNO3 |
| Molar mass | 251.045100 g/mol |
| 3D model (JSmol) | |
| |
| |
Crisaborole (trade name Eucrisa) is a nonsteroidal topical medication approved in the United States for the treatment of mild-to-moderate atopic dermatitis (eczema) in patients two years of age and older.[1][2] It was approved by U.S. Food and Drug Administration on Dec 14, 2016.[3]
Mechanism of action
It is a phosphodiesterase-4 inhibitor, mainly acting on phosphodiesterase 4B (PDE4B), which causes inflammation.[4] Chemically, crisaborole is a phenoxybenzoxaborole.[4] It contains a boron atom that helps penetrate the skin and is essential for its binding activity.[5] Inhibition of PDE4B appears to suppress the release of tumor necrosis factor alpha (TNFα), interleukin-12 (IL-12), IL-23 and other cytokines, proteins believed to be involved in the immune response and inflammation.[4]
Chemistry
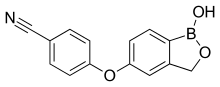
The chemical name for crisaborole is 4-[(1-hydroxy-1,3-dihydro-2,1-benzoxaborol-5-yl)oxy]benzonitrile.[6]
Drug development
Crisaborole was developed by Anacor Pharmaceuticals for the topical treatment of psoriasis.[7][8][4][9] During preclinical and clinical development, crisaborole was called AN2728 and PF-06930164.[10]
See also
- Phosphodiesterase
- Tavaborole (trade name Kerydin) – a structurally related topical antifungal developed by Anacor
References
- ↑ "FDA Approves Eucrisa for Eczema". U.S. Food and Drug Administration. 14 December 2016.
- ↑ "Eucrisa (crisaborole) Ointment, 2%, for Topical Use. Full Prescribing Information". Anacor Pharmaceuticals, Inc. Palo Alto, CA 94303 USA. Retrieved 17 December 2016.
- ↑ "FDA approves Eucrisa for eczema" (Press release). U.S. Food and Drug Administration. December 14, 2016.
- 1 2 3 4 Moustafa, F; Feldman, SR (16 May 2014). "A Review of Phosphodiesterase-Inhibition and the Potential Role for Phosphodiesterase 4-Inhibitors in Clinical Dermatology" (PDF). Dermatology Online Journal. 20 (5): 22608. PMID 24852768.
- ↑ Akama, T; Freund, Y; Kimura, R; Baker, S; Zhang, Y; Hernandez, V; Zhou, A; Sanders, V; Maples, KR; Plattner, J (April 2008). "Structure-Activity Studies of AN2728 and AN2898, Novel Oxaborole Compounds with Anti-Inflammatory Activities" (PDF). Conference Paper in Journal of Investigative Dermatology. Anacor Pharmaceuticals, Inc., 1020 East Meadow Circle, Palo Alto, CA 94043, USA. Archived from the original on 18 May 2015. Retrieved 17 December 2016.
- ↑ "WHO Drug Information, Vol. 29, No. 3, 2015. International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 74" (PDF). World Health Information. p. 391. Retrieved 26 April 2016.
- ↑ "Anacor Pharmaceuticals: Crisaborole". Anacor Pharmaceuticals, Inc. Retrieved 26 April 2016.
- ↑ Nazarian, R; Weinberg, JM (November 2009). "AN-2728, a PDE4 Inhibitor for the Potential Topical Treatment of Psoriasis and Atopic Dermatitis". Current Opinion in Investigational Drugs. 10 (11): 1236–42. PMID 19876791.
- ↑ Spreitzer, H (16 August 2016). "Neue Wirkstoffe: Crisaborol". Österreichische Apotheker-Zeitung (in German) (17/2016).
- ↑ "Crisaborole". AdisInsight. Retrieved 24 July 2017.