Avanafil
 | |
 Avanafil is a PDE5 inhibitor | |
| Clinical data | |
|---|---|
| Trade names | Stendra |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
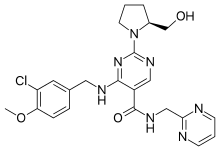
| Formula | C23H26ClN7O3 |
| Molar mass | 483.951 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Avanafil is a PDE5 inhibitor approved for erectile dysfunction by the FDA on April 27, 2012 [1] and by EMA on June 21, 2013.[2] Avanafil is known by the trademark names Stendra and Spedra. It was invented at Mitsubishi Tanabe Pharma, formerly known as Tanabe Seiyaku Co.[3] It was then out-licensed to, and developed by, Vivus Inc. In July 2013 Vivus announced partnership with Menarini Group, which will commercialise and promote Spedra in over 40 European countries plus Australia and New Zealand.[4] On Oct. 3, 2016 Vivus announced it had sold the exclusive rights to Avanafil in USA to Metuchen Pharmaceuticals LLC of New Jersey for an upfront payment of US$70,000,000.[5]
Avanafil acts by inhibiting a specific phosphodiesterase type 5 enzyme which is found in various body tissues, but primarily in the corpus cavernosum penis, as well as the retina.[6] Other similar drugs are sildenafil, tadalafil and vardenafil. The advantage of avanafil is that it has very fast onset of action compared with other PDE5 inhibitors. It is absorbed quickly, reaching a maximum concentration in about 30–45 minutes.[7] About two-thirds of the participants were able to engage in sexual activity within 15 minutes.[7]
Synthesis
Avanafil can be synthesized from a benzylamine derivative and a pyrimidine derivative:[8]
See also
- Tadalafil (Cialis)
- Vardenafil (Levitra/Staxyn)
- Sildenafil (Viagra)
References
- ↑ "FDA approves Stendra for erectile dysfunction" (Press release). Food and Drug Administration (FDA). April 27, 2012.
- ↑ "Spedra (avanafil)". European Medicines Agency. Retrieved 17 April 2014.
- ↑ https://patents.google.com/patent/US6656935B2
- ↑ http://ir.vivus.com/releasedetail.cfm?releaseid=775706
- ↑ http://ir.vivus.com/news-releases/news-release-details/vivus-and-metuchen-pharmaceuticals-announce-license-agreement
- ↑ "avanafil, Spedra". Medicine Net. Retrieved 17 April 2014.
- 1 2 Kyle, Jeffery; Brown, Dana (2013). "Avanafil for Erectile Dysfunction" (PDF). Annals of Pharmacotherapy. Sage Publishing. doi:10.1177/1060028013501989. Retrieved 28 September 2013.
- ↑ Yamada, K.; Matsuki, K.; Omori, K.; Kikkawa, K.; 2004, U.S. Patent 6,797,709
External links
- Faster-Working Erectile Dysfunction Drug?. CBS News. November 24, 2009.
- Vivus says men taking avanafil were more likely to be ready for sex within 15 minutes. The Gaea Times. January 11, 2010.
- "Avanafil is the New Player in The Erectile Dysfunction Field". June 28, 2011.
