Valvular heart disease
| Valvular heart disease | |
|---|---|
| Specialty | Cardiology |
Valvular heart disease is any disease process involving one or more of the four valves of the heart (the aortic and bicuspid valves on the left side of heart and the pulmonary and tricuspid valves on the right side of heart. These conditions occur largely as a consequence of aging,[1] but may also be the result of congenital (inborn) abnormalities or specific disease or physiologic processes including rheumatic heart disease and pregnancy.[2]
Anatomically, the valves are part of the dense connective tissue of the heart known as the cardiac skeleton and are responsible for the regulation of blood flow through the heart and great vessels. Valve failure or dysfunction can result in diminished heart functionality, though the particular consequences are dependent on the type and severity of valvular disease. Treatment of damaged valves may involve medication alone, but often involves surgical valve repair (valvuloplasty) or replacement (insertion of an artificial heart valve).
Types
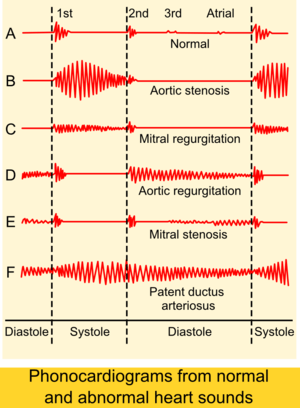
Stenosis and insufficiency/regurgitation represent the dominant functional and anatomic consequences associated with valvular heart disease. Irrespective of disease process, alterations to the valve occur that produce one or a combination of these conditions. Insufficiency and regurgitation are synonymous terms that describe an inability of the valve to prevent backflow of blood as leaflets of the valve fail to join (coapt) correctly. Stenosis is characterized by a narrowing of the valvular orifice that prevents adequate outflow of blood. Stenosis can also result in insufficiency if thickening of the annulus or leaflets results in inappropriate leaf closure.
| Valve involved | Stenotic disease | Insufficiency/regurgitation disease |
| Aortic valve | Aortic valve stenosis | Aortic insufficiency/regurgitation |
| Mitral valve | Mitral valve stenosis | Mitral insufficiency/regurgitation |
| Tricuspid valve | Tricuspid valve stenosis | Tricuspid insufficiency/regurgitation |
| Pulmonary valve | Pulmonary valve stenosis | Pulmonary insufficiency/regurgitation |
Aortic and mitral valve disorders
Aortic and mitral valve disease are termed left heart diseases. Diseases of these valves are more prevalent than disease of the pulmonary or tricuspid valve due to the higher pressures the left heart experiences.[3]
Stenosis of the aortic valve is characterized by a thickening of the valvular annulus or leaflets that limits the ability of blood to be ejected from the left ventricle into the aorta. It is typically the result of aging, occurring in 12.4% of the population over 75 years of age and represents the most common cause of outflow obstruction in the left ventricle.[1] Stenosis is typically the result of valvular calcification, but may be the result of a congenitally malformed bicuspid aortic valve. This defect is characterized by the presence of only two valve leaflets and affects up to 1% of the population, making it one of the most common cardiac abnormalities.[4] It may occur in isolation or in concert with other cardiac anomalies.
Aortic insufficiency, or regurgitation, is characterized by an inability of the valve leaflets to appropriately close at end systole, thus allowing blood to flow inappropriately backwards into the left ventricle. Causes of aortic insufficiency in the majority of cases are unknown, or idiopathic.[5] It may be the result of connective tissue or immune disorders, such as Marfan syndrome or systemic lupus erythematosus, respectively. Processes that lead to aortic insufficiency usually involve dilation of the valve annulus, thus displacing the valve leaflets, which are anchored in the annulus.
Mitral stenosis is caused largely by rheumatic heart disease, though is rarely the result of calcification. In some cases vegetations form on the mitral leaflets as a result of endocarditis, an inflammation of the heart tissue. Mitral stenosis is uncommon and not as age dependent as other types of valvular disease.[1]
Mitral insufficiency can be caused by dilation of the left heart, often a consequence of heart failure. In these cases the left ventricle of the heart becomes enlarged and causes displacement of the attached papillary muscles, which control the mitral valve. Mitral insufficiency is significantly associated with normal aging, rising in prevalence with age. It is estimated to be present in over 9% of people over 75.[1]
Pulmonary and tricuspid valve disorders
Pulmonary and tricuspid valve diseases are right heart diseases. Pulmonary valve diseases are the least common heart valve disease in adults.[1][3]
Pulmonary valve stenosis is often the result of congenital malformations and is observed in isolation or as part of a larger pathologic process, as in Tetralogy of Fallot, Noonan syndrome, and congenital rubella syndrome . Unless the degree of stenosis is severe individuals with pulmonary stenosis usually have excellent outcomes and treatment options. Often patients do not require intervention until later in adulthood as a consequence of calcification that occurs with aging.
Pulmonary valve insufficiency occurs commonly in healthy individuals to a very mild extent and does not require intervention.[6] More appreciable insufficiency it is typically the result of damage to the valve due to cardiac catheterization, aortic balloon pump insertion, or other surgical manipulations. Additionally, insufficiency may be the result of carcinoid syndrome, inflammatory processes such a rheumatoid disease or endocarditis, or congenital malformations.[7][8] It may also be secondary to severe pulmonary hypertension.
Tricuspid valve stenosis without co-occurrent regurgitation is highly uncommon and typically the result of rheumatic disease. It may also be the result of congenital abnormalities, carcinoid syndrome, obstructive right atrial tumors (typically lipomas or myxomas), or hypereosinophilic syndromes.
Minor tricuspid insufficiency is common in healthy individuals.[9] In more severe cases it is a consequence of dilation of the right ventricle, leading to displacement of the papillary muscles which control the valve's ability to close.[10] Dilation of the right ventricle occurs secondary to ventricular septal defects, right to left shunting of blood, eisenmenger syndrome, hyperthyroidism, and pulmonary stenosis. Tricuspid insufficiency may also be the result of congenital defects of the tricuspid valve, such as Ebstein's anomaly.
Dysplasia
Heart valve dysplasia is an error in the development of any of the heart valves, and a common cause of congenital heart defects in humans as well as animals; tetralogy of Fallot is a congenital heart defect with four abnormalities, one of which is stenosis of the pulmonary valve. Ebstein's anomaly is an abnormality of the tricuspid valve.[11]
Inflammatory disorders
Inflammation of the heart valves due to any cause is called valvular endocarditis; this is usually due to bacterial infection but may also be due to cancer (marantic endocarditis), certain autoimmune conditions (Libman-Sacks endocarditis, seen in systemic lupus erythematosus) and hypereosinophilic syndrome (Loeffler endocarditis). Certain medications have been associated with valvular heart disease, most prominently ergotamine derivatives pergolide and cabergoline.[12]
Valvular heart disease resulting from rheumatic fever is referred to as "rheumatic heart disease". Damage to the heart valves follows infection with beta-hemolytic bacteria, such as typically of the respiratory tract. Pathogenesis is dependent on cross reaction of M proteins produced by bacteria with the myocardium.[13] This results in generalized inflammation in the heart, this manifests in the mitral valve as vegetations, and thickening or fusion of the leaflets, leading to a severely compromised buttonhole valve.
Rheumatic heart disease typically only involves the mitral valve (70% of cases), though in some cases the aortic and mitral valves are both involved (25%). Involvement of other heart valves without damage to the mitral are exceedingly rare.[13]
While developed countries once had a significant burden of rheumatic fever and rheumatic heart disease, medical advances and improved social conditions have dramatically reduced their incidence. Many developing countries, as well as indigenous populations within developed countries, still carry a significant burden of rheumatic fever and rheumatic heart disease and there has been a resurgence in efforts to eradicate the diseases in these populations.
In pregnancy
The evaluation of individuals with valvular heart disease who are or wish to become pregnant is a difficult issue. Issues that have to be addressed include the risks during pregnancy to the mother and the developing fetus by the presence of maternal valvular heart disease as an intercurrent disease in pregnancy. Normal physiological changes during pregnancy require, on average, a 50% increase in circulating blood volume that is accompanied by an increase in cardiac output that usually peaks between the midportion of the second and third trimesters.[14] The increased cardiac output is due to an increase in the stroke volume, and a small increase in heart rate, averaging 10 to 20 beats per minute.[14] Additionally uterine circulation and endogenous hormones cause systemic vascular resistance to decrease and a disproportionately lowering of diastolic blood pressure causes a wide pulse pressure.[14] Inferior vena caval obstruction from a gravid uterus in the supine position can result in an abrupt decrease in cardiac preload, which leads to hypotension with weakness and lightheadedness.[14] During labor and delivery cardiac output increases more in part due to the associated anxiety and pain, as well as due to uterine contractions which will cause an increases in systolic and diastolic blood pressure.[14]
Valvular heart lesions associated with high maternal and fetal risk during pregnancy include:[14]
- Severe aortic stenosis with or without symptoms
- Aortic regurgitation with NYHA functional class III-IV symptoms
- Mitral stenosis with NYHA functional class II-IV symptoms
- Mitral regurgitation with NYHA functional class III-IV symptoms
- Aortic and/or mitral valve disease resulting in severe pulmonary hypertension (pulmonary pressure greater than 75% of systemic pressures)
- Aortic and/or mitral valve disease with severe LV dysfunction (EF less than 0.40)
- Mechanical prosthetic valve requiring anticoagulation
- Marfan syndrome with or without aortic regurgitation
In individuals who require an artificial heart valve, consideration must be made for deterioration of the valve over time (for bioprosthetic valves) versus the risks of blood clotting in pregnancy with mechanical valves with the resultant need of drugs in pregnancy in the form of anticoagulation.
Comparison
The following table includes the main types of valvular stenosis and regurgitation. Major types of valvular heart disease not included in the table include mitral valve prolapse, rheumatic heart disease and endocarditis.
| Valvular disease | Mitral stenosis | Aortic stenosis | Aortic regurgitation | Mitral regurgitation | Tricuspid regurgitation |
|---|---|---|---|---|---|
| Prevalence | Most common valvular heart disease in pregnancy[15] | Approximately 2% of people over the age of 65, 3% of people over age 75, and 4% percent of people over age 85[16] | 2% of the population, equally in males and females.[17] | ||
| Main causes and risk factors | Almost always caused by rheumatic heart disease[18] |
Hypertension, diabetes mellitus, hyperlipoproteinemia and uremia may speed up the process.[19] |
Acute
Chronic
|
Acute
Chronic |
|
| Hemo dynamics / Patho- physiology |
Progressive obstruction of the mitral ostium causes increased pressure in the left atrium and the pulmonary circulation.[19] Congestion may cause thromboembolism, and atrial hypertension may cause atrial fibrillation.[19] | Obstruction through the aortic ostium causes increased pressure in the left ventricle and impaired flow through the aorta | Insufficiency of the aortic valve causes backflow of blood into the left ventricle during diastole. | Insufficiency of the mitral valve causes backflow of blood into the left atrium during systole. | Insufficiency of the tricuspid valve causes backflow of blood into the right atrium during systole. |
| Symptoms |
Symptoms increase with exercise and pregnancy[18] |
|
|
|
|
| Medical signs |
Signs increase with exercise and pregnancy[18] |
|
|
In acute cases, the murmur and tachycardia may be only distinctive signs.[19] |
|
| Diagnosis |
|
|
|
| |
| Treatment |
No therapy is required for asymptomatic patients. Diuretics for any pulmonary congestion or edema.[18] If stenosis is severe, surgery is recommended.[18] Any atrial fibrillation is treated accordingly.[18]
|
No treatment in asymptomatic patients.[18]
Medical therapy and percutaneous balloon valvuloplasty have relatively poor effect.[18]
|
Also, endocarditis prophylaxis is indicated before dental, gastrointestinal or genitourinary procedures.[18] |
|
|
| Follow-up |
|
References
- 1 2 3 4 5 Burden of valvular heart diseases: a population-based study. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano. Lancet. 2006 Sep;368(9540):1005-11.
- ↑ Pregnancy and contraception in congenital heart disease: what women are not told. Kovacs AH, Harrison JL, Colman JM, Sermer M, Siu SC, Silversides CK J Am Coll Cardiol. 2008;52(7):577.
- 1 2 Ragavendra R. Baliga, Kim A. Eagle, William F Armstrong, David S Bach, Eric R Bates, Practical Cardiology, Lippincott Williams & Wilkins, 2008, page 452.
- ↑ Braverman AC. The Bicuspid Aortic Valve and Associated Aortic Disease. In: Valvular Heart Disase, 4th, Otto CM, Bonow RO. (Eds), Saunders/Elsevier, Philadelpha 2013. p.179.
- ↑ Chapter 1: Diseases of the Cardiovascular system > Section: Valvular Heart Disease in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
- ↑ 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. J Am Coll Cardiol. 2014;63(22):e57.
- ↑ Isolated pulmonic valve infective endocarditis: a persistent challenge. Hamza N, Ortiz J, Bonomo. Infection. 2004 Jun;32(3):170-5.
- ↑ Carcinoid heart disease. Clinical and echocardiographic spectrum in 74 patients. Pellikka PA, Tajik AJ, Khandheria BK, Seward JB, Callahan JA, Pitot HC, Kvols LK. Circulation. 1993;87(4):1188.
- ↑ Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ, American Society of Echocardiography. J Am Soc Echocardiogr. 2003;16(7):777.
- ↑ Impact of tricuspid regurgitation on long-term survival. Nath J, Foster E, Heidenreich PA. J Am Coll Cardiol. 2004;43(3):405.
- ↑ Bonow RO, Carabello BA, Kanu C, et al. (2006). "ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons". Circulation. 114 (5): e84–231. doi:10.1161/CIRCULATIONAHA.106.176857. PMID 16880336.
- ↑ Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (2007). "Dopamine agonists and the risk of cardiac-valve regurgitation". N. Engl. J. Med. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453. Archived from the original on 2007-04-22.
- 1 2 Vinay, Kumar (2013). Robbin's Basic Pathology. Elsevier Health Sciences. pp. Chapter 10: Heart.
- 1 2 3 4 5 6 7 Bonow, RO; Carabello, BA; Chatterjee, K; De Leon Jr, AC; Faxon, DP; Freed, MD; Gaasch, WH; Lytle, BW; et al. (2008). "2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Journal of the American College of Cardiology. 52 (13): e1–142. doi:10.1016/j.jacc.2008.05.007. PMID 18848134.
- ↑ Gelson, E.; Gatzoulis, M.; Johnson, M. (2007). "Valvular heart disease". BMJ (Clinical research ed.). 335 (7628): 1042–1045. doi:10.1136/bmj.39365.655833.AE. PMC 2078629. PMID 18007005.
- ↑ Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997; 29: 630-634.
- ↑ The Cleveland Clinic Center for Continuing Education > Mitral Valve Disease: Stenosis and Regurgitation Archived 2010-09-21 at the Wayback Machine. Authors: Ronan J. Curtin and Brian P. Griffin. Retrieved September 2010
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 Chapter 1: Diseases of the Cardiovascular system > Section: Valvular Heart Disease in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 VOC=VITIUM ORGANICUM CORDIS, a compendium of the Department of Cardiology at Uppsala Academic Hospital. By Per Kvidal September 1999, with revision by Erik Björklund May 2008
External links
| Classification |
|---|