Catecholaminergic polymorphic ventricular tachycardia
| Catecholaminergic polymorphic ventricular tachycardia | |
|---|---|
| Synonyms | CPVT |
| Specialty | Cardiology |
| Symptoms | Blackouts, sudden cardiac death |
| Usual onset | Childhood / adolescence |
| Causes | Genetic |
| Risk factors | Family history |
| Diagnostic method | Electrocardiogram (ECG), genetic testing, adrenaline provocation, exercise testing |
| Differential diagnosis | Long QT syndrome, Brugada syndrome, Andersen-Tawil syndrome Early repolarisation syndrome |
| Treatment | Avoidance of strenuous exercise, medication, implantable cardioverter defibrillator |
| Medication | Beta-adrenoceptor blockers, Verapamil, Flecainide |
| Prognosis | 13-20% life threatening arrhythmias over 7-8 years |
| Frequency | 1:10,000 |
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited disorder that predisposes those affected to potentially life-threatening abnormal heart rhythms or arrhythmias. The arrhythmias seen in CPVT typically occur during exercise or at times of emotional stress, and typically take the form of bidirectional ventricular tachycardia or ventricular fibrillation. Those affected may be asymptomatic, but may experience blackouts or even sudden cardiac death.
CPVT is caused by genetic mutations affecting proteins that regulate the concentrations of calcium within cardiac muscle cells. The most commonly identified gene is RYR2, encoding a protein encoding a channel known as the ryanodine receptor, which releases calcium from the cells internal calcium store, the sarcoplasmic reticulum, during every heartbeat.
CPVT is often diagnosed on an ECG recorded during an exercise tolerance test, but may also be diagnosed with a genetic test. The condition is treated with medication including beta-adrenoceptor blockers or flecainide, and with surgical procedures including sympathetic denervation and implantation of a defibrillator.
The condition is thought to affect as many as one in ten thousand people and is estimated to cause 15% of all unexplained sudden cardiac deaths in young people. CPVT was first recognised in 1960, and the underlying genetics were described in 2001.
Signs and symptoms
.jpg)
Although patients with CPVT may not have any symptoms, the most commonly reported symptoms in persons with CPVT are blackouts or sudden loss of consciousness, referred to as syncope.[1] These blackouts often occur during exercise or as a response to emotional stress - situations when chemical messengers known as catecholamines such as adrenaline are released within the body. In those with CPVT, catecholamine release can lead to an abnormal heart rhythm or arrhythmia known as ventricular tachycardia. [2] In CPVT, the ventricular tachycardia often takes a characteristic form known as bidirectional ventricular tachycardia.[1] This arrhythmia in some cases terminates by itself, causing a blackout from which the person then recovers. However, if the abnormal heart rhythm continues, it can degenerate into a more dangerous arrhythmia known as ventricular fibrillation causing a cardiac arrest, and if untreated, sudden death. Sudden death may be the first manifestation of the disease in some patients, which may take the form of sudden infant death syndrome or 'cot death'.[1]
There are typically very few abnormal signs on clinical examination in persons with CPVT. However, approximately 20% of those with CPVT have a slow resting heart rate known as a sinus bradycardia.[2]
Mechanism
Excitation contraction coupling
The arrhythmias that those with CPVT experience are caused by abnormalities in the way that cardiac muscle cells control their levels of calcium. [3] Calcium interacts with the protein fibres or myofibrils inside the cell that allow the cell to contract, and the concentration of calcium within each cell needs to be very tightly regulated. During each heartbeat, the concentration of calcium must rise to allow the muscle to contract, and then fall to allow the muscle to relax, a process achieved by using a store within the cell known as the sarcoplasmic reticulum.[4]
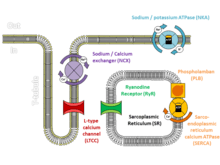
At the start of each heartbeat, calcium is released from the sarcoplasmic reticulum through specialised channels known as ryanodine receptors. [4] In response to an electrical signal from the cell membrane called an action potential, a small amount of calcium flowing across the cell membrane triggers ryanodine receptors to release a puff of calcium known as a calcium spark. Each spark triggers the release of further sparks from neighbouring ryanodine receptors to create an organised rise of calcium throughout the cell known as a calcium transient. At the end of each heartbeat, calcium is pumped back by a protein called SERCA, and held within the sarcoplasmic reticulum by a protein called calsequestrin.[4] Alterations to the proteins involved in this mechanism can disrupt this carefully regulated process and lead to arrhythmias.
Calcium-dependent arrhythmias
In those with CPVT, the normally tight regulation of calcium can become deranged.[3] While calcium is generally released from the sarcoplasmic reticulum response to an action potential, calcium sparks can occur spontaneously in healthy and diseased hearts. In a healthy heart, a spontaneous calcium spark is generally an isolated event and goes no further, but if ryanodine receptors or the proteins that regulate them are abnormal, these sparks can trigger releases from neighbouring ryanodine receptors which spread throughout the cell as a calcium wave.[3] These calcium waves are much more likely to occur when cardiac muscle cells are stimulated by catecholamines such as adrenaline which increase the concentration of calcium within the sarcoplasmic reticulum and sensitise the ryanodine receptors. The uncontrolled wave of calcium can be forced out through the cell membrane, causing an electrical current known as a delayed afterdepolarisation. Afterdepolarisations, if large enough, can trigger additional action potentials, premature ventricular contractions, or sustained arrhythmias.[3]
Molecular genetics
CPVT can be caused by mutations in several genes, all of which are responsible for regulating the concentrations of calcium within cardiac muscle cells. The most commonly identified genetic mutation in CPVT is a mutation in the RYR2 gene that encodes the cardiac ryanodine receptor, responsible for releasing calcium from the sarcoplasmic reticulum.[5] Mutations in this gene lead to an autosomal dominant form of CPVT. Mutations associated with CPVT have also been identified in the CASQ2 gene which encodes calsequestrin, a protein that binds calcium within the sarcoplasmic reticulum.[5] Other genes associated with CPVT include TECRL encoding Trans-2,3-enoyl-CoA reductase-like protein, CALM1 encoding Calmodulin, and TRDN encoding Triadin.[5]
| Type | OMIM | Gene | Locus | Inheritance | Notes |
|---|---|---|---|---|---|
| CPVT1 | 604772 | RYR2 | 1q42.1-q43 | AD | Ryanodine receptor - releases calcium from the sarcoplasmic reticulum [5] |
| CPVT2 | 611938 | CASQ2 | 1p13.3-p11 | AR | Calsequestrin - calcium buffer within the sarcoplasmic reticulum [5] |
| CPVT3 | 614021 | TECRL | 7p22-p14 | AR | Trans-2,3-enoyl-CoA reductase-like protein - interacts with RyR2 and calsequestrin [5] |
| CPVT4 | 614916 | CALM1 | 14q32.11 | AD | Calmodulin - stabilises RyR2 [5] |
| CPVT5 | 615441 | TRDN | 6q22.31 | AR | Triadin - interacts with RyR2 and calsequestrin [5] |
CPVT1 (RYR2)
Mutation of the Ryanodine receptor isoform 2 (RYR2) gene has been linked to catecholaminergic polymorphic ventricular tachycardia (CPVT).[6] Under normal physiological conditions, RYR2 mutation has no discernable effect on calcium induced-calcium release from the sarcoplasmic reticulum (SR).[6] Ryr2 is normally activated by increased cytosolic calcium, but under stressful conditions such as increased beta adrenergic activation, RYR2 is activated by luminal calcium in association with increased SR calcium loading.[6][7][8] The increased luminal calcium activation occurs because of a phenomenon termed store-overload induced calcium release (SOICR).[9] SOICR leads to spontaneous and inappropriate action potentials, generating arrhythmias.[10][11][12] A Ryr2 mutation may increase sensitivity to luminal calcium activation, therefore increasing calcium release from the SR under store-overload conditions and thus triggered arrhythmias.[13][14]
RYR2 mutations have been well characterized and been found to occur primarily in 4 major domains.[6] Mutations in domains III and IV of the protein (amino acid range from 3778 to 4201 and 4497 to 4959 respectively) occur in 46% of reported mutations.[6] Mutations occur less frequently in domains I and II (amino acid 77-466 and 2246-2534 respectively).[6] Causative RYR2 mutations outside these four domains are very rare, occurring in as little as 10% of reported cases.[15] Ryr2 mutations are most often single nucleotide substitutions resulting in a different amino acid substitution, however some in-frame substitutions and duplications have been documented [15][16] . It is commonly accepted that more severe mutations have not been linked to CPTV as they are more likely to underlie different cardiac pathologies.[6]
Recent findings have characterized the pathology of RYR2 mutations and how they relate to SOICR as a matter of the intrinsic properties of the ryanodine channel. Two theories propose the underlying mechanism, domain unzipping and FKBP12.6 unbinding.[6] Firstly, domain unzipping refers to the separation of the N-terminal domain's interaction with the central domain; destabilizing the receptor.[17][18] The mutation would compromise the stability of the Ryr2's closed state and increase its sensitivity to stimuli like luminal and cytosolic calcium.[6][17][18] Domain unzipping coincides with the specific Ryr2 domain mutations associated with CPTV.[19] The second theory of FKBP12.6 is more controversial.[19] FKBP12.6 is a RYR2 binding protein that stabilizes the receptor. FKBP12.6 binding to RYR2 is regulated by RYR2 phosphorylation via PKA that results in the dissociation of FKBP12.6, rendering Ryr2 more sensitive to cytosolic calcium activation.[20] However, as mentioned above, evidence has been conflicted in determining FKBP12.6's role in CPTV.[6] So far the literature concludes that FKBP12.6 may play a role in certain CPTV mutations but not others, further research needs to clarify this protein's role.[6]
CPVT2 (CASQ2)
Mutations in the Calsequestrin isoform 2 (CASQ2) gene has been linked to CPVT.[21] Under normal physiological conditions, CASQ2 is the major luminal Ca2+ binding protein in the sarcoplasmic reticulum (SR)[6][21][22][23][24][25][26] ), which in the main Ca2+ storage organelle in cardiac muscle. CASQ2 is also associated with regulating SR Ca2+ release when bound to triadin, junctin and RYR2, forming a complex [22] .[21] This cytosolic to luminal Ca2+ activation process that RYR2 regulates is termed store-overload induced calcium release (SOICR). CASQ2 is responsible for initiating and terminating this process.[6] CASQ2 acts in low levels of SR Ca2+, where CASQ2 monomers inhibit RYR2 by forming the triadin-junctin-RYR2 complex, however at high levels of SR Ca2+, CASQ2 monomers form polymers and dissociate from the RYR2 channel complex, removing the inhibitory response activating the channel to spontaneously release Ca2+.[6][24] A mutation, specifically R33Q and D307H in CASQ2 tend to alter the Ca2+ binding capacity or alter the interactions between CASQ2 and RYR2 channel complex, potentially affecting the response of RYR2.[6][24]
Mutations in the CASQ2 gene have been classified into 12 CPVT associated mutations: 4 are nonsense mutations causing shortening of proteins, and 8 are missense mutations. R33Q and D307H reduce CASQ2 protein to 5% and 45% of normal levels respectively, which reduces SR Ca2+ buffering and binding capacity.[6][22][25][26] The most severe missense mutation, D307H, converts aspartic acid (negatively charged) to a histidine within a Ca2+ chelating region. This disrupts Ca2+ binding to CASQ2, but the specific mechanism behind this mutation is still undetermined.[25][26] The missense mutation R33Q causes a substitution of glutamine for arginine, decreasing the total amount of Ca2+ stored in the SR, thus increasing the Ca2+ buffering system causing Ca2+ leak through RYR2, where the mechanism behind this mutation is proposed to interact with triadin and/or junction forming "polar zippers".[24][25]
There are two major theories as to what is occurring when CASQ2 is deficient. It was found that decreased CASQ2 is associated with high levels of calreticulin (CRT).[22] In the absence of CASQ2 signal, CRT levels increase and provide some compensatory SR Ca2+ binding activity. CRT levels decrease significantly after birth and high levels are only present in the developing heart, leading to the theory of caused bradycardia and sinus node dysfunction which is found in CPTV patients.[22] With the absence of CASQ2, it was also found that RYR2 activity remained high in diastole since CASQ2 could not provide the inhibitory response, causing a prolonged Ca2+ leak which triggers early action potentials.[21][22][23] With reduced SR Ca2+ buffering capacity, is a faster recovery of SR free Ca2+ after each Ca2+ release, resulting in higher levels of SR free Ca2+ and SR Ca2+ loading, both increasing trigger activity and SOICR recurrence.[6][22] The exact mechanisms by which the mutations occur in the CASQ2 gene are still under investigation. Research underway is analyzing strategies to target RYR2 inhibition and approaches to increasing SR Ca2+.[22]
Diagnosis
CPVT may be challenging to diagnose. The structure of the heart appears normal in those affected by the condition when assessed using an echocardiogram, cardiac MRI scan or cardiac CT scan. At rest, even the electrical function of the heart appears normal when assessed using a standard 12-lead ECG.[27] However, in response to exercise or catecholamines such as adrenaline, abnormal heart rhythms such as bidirectional ventricular tachycardia or frequent polymorphic ventricular ectopic beats may be seen.[28]
12-lead ECG
.jpg)
The resting 12-lead ECG is a useful test to differentiate CPVT from other electrical diseases of the heart that can cause similar abnormal heart rhythms. Unlike conditions such as long QT syndrome and Brugada syndrome, the resting 12-lead ECG in those with CPVT is generally normal. However, approximately 20% of those affected have a slow resting heart rate or sinus bradycardia.[5]
Exercise and other provocative testing
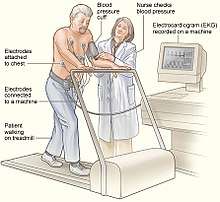
Exercise testing, commonly performed on a treadmill or stationary bicycle, can help to diagnose CPVT. During the test, those with CPVT often experience ectopic beats, which may progress to bidirectional and then polymorphic ventricular tachycardia as the intensity of exercise increases.[29] Some of those suspected of having CPVT, such as young children, may not be able to perform an exercise tolerance test. In these cases, alternative forms of testing include adrenaline provocation testing, during which adrenaline is infused into a vein at gradually increasing doses under close supervision and ECG monitoring.[28] Additionally, long term or Holter ECG monitoring can be performed, although this form of testing is less likely to detect an arrhythmia. Invasive electrophysiological studies do not provide useful information to help diagnose CPVT or to assess the risk of life threatening arrhythmias.[27][28]
Genetic testing
CPVT can also be diagnosed by identifying a disease-causing mutation in a gene associated with CPVT using genetic testing.[28][27] This technique may be the only way to identify the cause of death in someone suspected of having CPVT, and in this case may be known as a molecular autopsy.[30]
Treatment
The treatment for CPVT aims to prevent lethal abnormal heart rhythms from occurring, and to rapidly restore a normal rhythm if they do occur. As the arrhythmias in CPVT generally occur at times when the heart is exposed to high levels of adrenaline or other similar chemical messengers (catecholamines), many treatments for CPVT aim to lower the levels of catecholamines the heart is exposed to or block their effects on the heart.
The first line treatment for those with CVT involves lifestyle advice. This includes avoiding competitive sports,very strenuous exercise and highly stressful environments as high levels of adrenaline can occur in these settings which can provoke arrhythmias.[27]
Medication
Several medications can be useful for those with CPVT. The mainstays of treatment are beta blockers which block the effects of adrenaline on the heart, reducing the chance of abnormal heart rhythms developing.[27] Of all the beta blockers, Nadolol may be the most effective for treating CPVT.[5] This drug lowers the heart rate to a greater extent than other beta blockers and only needs to be taken once daily, reducing the chance of missed doses.[5] Propranolol is an alternative beta blocker as Nadolol is not available in all countries.[5]
Flecainide is a class 1c antiarrhythmic drug that is recommended for those with CPVT who experience abnormal heart rhythms despite taking a beta blocker.[27] Flecainide reduces the risk of arrhythmias in those with CPVT, but it remains uncertain how Flecainide achieves this. Some have suggested that Flecainide directly interacts with the cardiac ryanodine receptor which is frequently abnormal in those with CPVT, while other suggest that the anti-arrhythmic effects of Flecainide rely entirely on its sodium channel blocking effects.[31]
Verapamil is a calcium channel antagonist that, when combined with a beta blocker, may reduce the risk of arrhythmias in patients with CPVT.[32][33] Propafenone is another antiarrhythmic that may reduce the risk of arrhythmias, potentially through direct effects on the ryanodine receptor.[31]
Sympathetic denervation

Some persons with CPVT continue to experience life-threatening arrhythmias despite medical therapy. In this case a surgical procedure can be used to reduce the levels of adrenaline that the heart is exposed to.[27] A part of the sympathetic nervous system which supplies adrenaline to the body's organs can be intentionally damaged in an operation known as cardiac sympathetic denervation or sympathectomy.[34] While the sympathetic nervous system feeds into the heart from both sides, often only the left sided nerves are targeted during sympathectomy, although destruction of the nerves on both sides may be required.[34] By disrupting the supply of adrenaline to the heart, sympathectomy is effective at decreasing the risk of further life-threatening arrhythmias.[2]
Implantable cardioverter-defibrillator
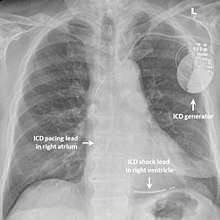
While medication and sympathectomy aim to prevent abnormal heart rhythms from occurring in the first place, an implantable defibrillator (ICD) may be used to treat arrhythmias that medication has failed to prevent and restore a normal heart rhythm.[27] These devices, usually implanted under the skin at the front of the chest below the shoulder, can continuously monitor the heart looking for abnormal heart rhythms. If a life-threatening arrhythmia is detected, the device can deliver a small electric shock to terminate the abnormal rhythm and restart the heart.[1]
Implantable defibrillators are often recommended for those with CPVT who have experienced blackouts, ventricular arrhythmias or cardiac arrest despite taking appropriate medication.[27] These devices can be life saving, although the resulting surge of adrenaline caused by the pain of an electric shock from the device can sometimes bring on a cycle of recurrent arrhythmias and shocks known as an electrical storm.[2] Because of this, it is strongly recommended that those with an ICD implanted for CPVT should take a beta blocker to dampen down the effects of adrenaline.[2]
Epidemiology
CPVT is estimated to affect 1 in 10,000 people.[2] Symptoms from CPVT are typically first seen in the first or second decade of life and more than 60% of affected individuals having their first episode of syncope or cardiac arrest by age 12-20.[1] However, a small number of patients may present later in life, and genetic testing in these patients frequently fails to identify a causative gene.[2]
Prognosis
A significant proportion of those with CPVT will experience a life-threatening abnormal heart rhythm, with estimates of this risk ranging from 13-20% over the course of 7–8 years.[35][35] Life-threatening arrhythmias are more likely to occur if CPVT has been diagnosed in childhood, if a person with CPVT does not take beta blockers, and if arrhythmias occur on exercise testing despite taking beta blockers.[27]
History
In 1960, Norwegian cardiologist Dr Knut Berg published a report on 3 sisters who suffered from blackouts during exercise or emotional stress in what is now recognised as the first description of CPVT.[5] The bidirectional ventricular tachycardia associated with this condition was described by Reid in 1975.[1] The term "Catecholaminergic Polymorphic Ventricular Tachycardia" was used in the case series published by Coumel in 1978 and Leenhardt in 1995.[36] In 1999, the first genetic mutation causing CPVT to be identified was localised to chromosome 1q42-q43,[37] which was found to be a variant in the RYR2 gene in 2001.[38] Ongoing research aims to identify better treatments for CPVT, increase understanding of the mechanisms of arrhythmia, and identify other genes causing the condition.[39]
See also
References
- 1 2 3 4 5 6 Liu N, Napolitano C, Priori S (2013). Chapter 31: Catecholaminergic Polymorphic Ventricular Tachycardia. In: Electrical diseases of the heart. Volume 2, Diagnosis and treatment. Arthur Wilde, Ihor Gussak, Michael J. Ackerman, Win-Kuang Shen, and Charles Antzelevitch (2nd ed.). London: Springer. ISBN 9781447149781. OCLC 846445829.
- 1 2 3 4 5 6 7 Obeyesekere MN, Antzelevitch C, Krahn AD (February 2015). "Management of ventricular arrhythmias in suspected channelopathies". Circulation: Arrhythmia and Electrophysiology. 8 (1): 221–31. doi:10.1161/CIRCEP.114.002321. PMID 25691556.
- 1 2 3 4 Venetucci, Luigi; Denegri, Marco; Napolitano, Carlo; Priori, Silvia G. (2012-10). "Inherited calcium channelopathies in the pathophysiology of arrhythmias". Nature Reviews. Cardiology. 9 (10): 561–575. doi:10.1038/nrcardio.2012.93. ISSN 1759-5010. PMID 22733215. Check date values in:
|date=(help) - 1 2 3 M., Bers, D. (2001). Excitation-contraction coupling and cardiac contractile force (2nd ed.). Dordrecht: Kluwer Academic Publishers. ISBN 0792371577. OCLC 47659382.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Lieve KV, van der Werf C, Wilde AA (May 2016). "Catecholaminergic Polymorphic Ventricular Tachycardia". Circulation Journal. 80 (6): 1285–91. doi:10.1253/circj.CJ-16-0326. PMID 27180891.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Priori SG, Chen SR (April 2011). "Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis". Circulation Research. 108 (7): 871–83. doi:10.1161/circresaha.110.226845. PMC 3085083. PMID 21454795.
- ↑ Lakatta EG (March 1992). "Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart". Cardiovascular Research. 26 (3): 193–214. doi:10.1093/cvr/26.3.193. PMID 1423412.
- ↑ Lakatta, E. G.; Guarnieri T. (1993). "Spontaneous myocardial calcium oscillations: are they linked to ventricular fibrillation?". Journal of Cardiovascular Electrophysiology. 4 (473–489).
- ↑ Laver DR (May 2007). "Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites". Biophysical Journal. 92 (10): 3541–55. Bibcode:2007BpJ....92.3541L. doi:10.1529/biophysj.106.099028. PMC 1853142. PMID 17351009.
- ↑ Bers DM (January 2002). "Calcium and cardiac rhythms: physiological and pathophysiological". Circulation Research. 90 (1): 14–7. PMID 11786512.
- ↑ Pogwizd SM, Bers DM (February 2004). "Cellular basis of triggered arrhythmias in heart failure". Trends in Cardiovascular Medicine. 14 (2): 61–6. doi:10.1016/j.tcm.2003.12.002. PMID 15030791.
- ↑ Schlotthauer K, Bers DM (October 2000). "Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials". Circulation Research. 87 (9): 774–80. doi:10.1161/01.res.87.9.774. PMID 11055981.
- ↑ Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR (November 2005). "Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death". Circulation Research. 97 (11): 1173–81. doi:10.1161/01.res.0000192146.85173.4b. PMID 16239587.
- ↑ Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR (August 2004). "RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR)". Proceedings of the National Academy of Sciences of the United States of America. 101 (35): 13062–7. Bibcode:2004PNAS..10113062J. doi:10.1073/pnas.0402388101. PMC 516517. PMID 15322274.
- 1 2 Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JP, Mannens MM, Wilde AA, Ackerman MJ (November 2009). "The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis". Journal of the American College of Cardiology. 54 (22): 2065–74. doi:10.1016/j.jacc.2009.08.022. PMC 2880864. PMID 19926015.
- ↑ Tester DJ, Kopplin LJ, Will ML, Ackerman MJ (October 2005). "Spectrum and prevalence of cardiac ryanodine receptor (RyR2) mutations in a cohort of unrelated patients referred explicitly for long QT syndrome genetic testing". Heart Rhythm. 2 (10): 1099–105. doi:10.1016/j.hrthm.2005.07.012. PMID 16188589.
- 1 2 Ikemoto N, Yamamoto T (October 2000). "Postulated role of inter-domain interaction within the ryanodine receptor in Ca(2+) channel regulation". Trends in Cardiovascular Medicine. 10 (7): 310–6. doi:10.1016/s1050-1738(01)00067-6. PMID 11343972.
- 1 2 Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, Uchinoumi H, Okuda S, Oda T, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M (February 2009). "Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts". Cardiovascular Research. 81 (3): 536–45. doi:10.1093/cvr/cvn303. PMC 2721653. PMID 18996969.
- 1 2 Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR (June 2003). "FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death". Cell. 113 (7): 829–40. doi:10.1016/s0092-8674(03)00434-3. PMID 12837242.
- ↑ Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR (May 2000). "PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts". Cell. 101 (4): 365–76. doi:10.1016/s0092-8674(00)80847-8. PMID 10830164.
- 1 2 3 4 Faggioni M, Kryshtal DO, Knollmann BC (August 2012). "Calsequestrin mutations and catecholaminergic polymorphic ventricular tachycardia". Pediatric Cardiology. 33 (6): 959–67. doi:10.1007/s00246-012-0256-1. PMC 3393815. PMID 22421959.
- 1 2 3 4 5 6 7 8 Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG (July 2007). "Calsequestrin 2 (CASQ2) mutations increase expression of calreticulin and ryanodine receptors, causing catecholaminergic polymorphic ventricular tachycardia". The Journal of Clinical Investigation. 117 (7): 1814–23. doi:10.1172/jci31080. PMC 1904315. PMID 17607358.
- 1 2 Liu N, Priori SG (January 2008). "Disruption of calcium homeostasis and arrhythmogenesis induced by mutations in the cardiac ryanodine receptor and calsequestrin". Cardiovascular Research. 77 (2): 293–301. doi:10.1093/cvr/cvm004. PMID 18006488.
- 1 2 3 4 Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, Napolitano C, Williams SC, Volpe P, Priori SG, Gyorke S (May 2006). "Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death". Circulation Research. 98 (9): 1151–8. doi:10.1161/01.res.0000220647.93982.08. PMID 16601229.
- 1 2 3 4 Viatchenko-Karpinski S, Terentyev D, Györke I, Terentyeva R, Volpe P, Priori SG, Napolitano C, Nori A, Williams SC, Györke S (March 2004). "Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin". Circulation Research. 94 (4): 471–7. doi:10.1161/01.res.0000115944.10681.eb. PMID 14715535.
- 1 2 3 Lahat H, Pras E, Olender T, Avidan N, Ben-Asher E, Man O, Levy-Nissenbaum E, Khoury A, Lorber A, Goldman B, Lancet D, Eldar M (December 2001). "A missense mutation in a highly conserved region of CASQ2 is associated with autosomal recessive catecholamine-induced polymorphic ventricular tachycardia in Bedouin families from Israel". American Journal of Human Genetics. 69 (6): 1378–84. doi:10.1086/324565. PMC 1235548. PMID 11704930.
- 1 2 3 4 5 6 7 8 9 10 Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ (November 2015). "2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC)". European Heart Journal. 36 (41): 2793–2867. doi:10.1093/eurheartj/ehv316. PMID 26320108.
- 1 2 3 4 Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C (December 2013). "HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013". Heart Rhythm. 10 (12): 1932–63. doi:10.1016/j.hrthm.2013.05.014. PMID 24011539.
- ↑ Obeyesekere MN, Klein GJ, Modi S, Leong-Sit P, Gula LJ, Yee R, Skanes AC, Krahn AD (December 2011). "How to perform and interpret provocative testing for the diagnosis of Brugada syndrome, long-QT syndrome, and catecholaminergic polymorphic ventricular tachycardia". Circulation: Arrhythmia and Electrophysiology. 4 (6): 958–64. doi:10.1161/CIRCEP.111.965947. PMID 22203660.
- ↑ Semsarian C, Ingles J (October 2016). "Molecular autopsy in victims of inherited arrhythmias". Journal of Arrhythmia. 32 (5): 359–365. doi:10.1016/j.joa.2015.09.010. PMC 5063264. PMID 27761159.
- 1 2 Lieve KV, Wilde AA, van der Werf C (May 2016). "The Role of Flecainide in the Management of Catecholaminergic Polymorphic Ventricular Tachycardia". Arrhythmia & Electrophysiology Review. 5 (1): 45–9. doi:10.15420/aer.2016.3.3. PMC 4939313. PMID 27403293.
- ↑ Rosso R, Kalman JM, Rogowski O, Diamant S, Birger A, Biner S, Belhassen B, Viskin S (September 2007). "Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia". Heart Rhythm. 4 (9): 1149–54. doi:10.1016/j.hrthm.2007.05.017. PMID 17765612.
- ↑ Sumitomo N (October 2016). "Current topics in catecholaminergic polymorphic ventricular tachycardia". Journal of Arrhythmia. 32 (5): 344–351. doi:10.1016/j.joa.2015.09.008. PMC 5063269. PMID 27761157.
- 1 2 Schwartz PJ, De Ferrari GM, Pugliese L (June 2017). "Cardiac sympathetic denervation 100years later: Jonnesco would have never believed it". International Journal of Cardiology. 237: 25–28. doi:10.1016/j.ijcard.2017.03.020. PMID 28318666.
- 1 2 Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A (May 2009). "Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia". Circulation. 119 (18): 2426–34. doi:10.1161/CIRCULATIONAHA.108.829267. PMID 19398665.
- ↑ Clinical cardiogenetics. Baars, H. F., Doevendans, Pieter A., Houweling, Arjan C., Tintelen, J. Peter van (Johannes Peter), 1964- (2nd ed.). Cham: Springer. 2016. ISBN 9783319442037. OCLC 965775116.
- ↑ Swan H, Piippo K, Viitasalo M, Heikkilä P, Paavonen T, Kainulainen K, Kere J, Keto P, Kontula K, Toivonen L (December 1999). "Arrhythmic disorder mapped to chromosome 1q42-q43 causes malignant polymorphic ventricular tachycardia in structurally normal hearts". Journal of the American College of Cardiology. 34 (7): 2035–42. PMID 10588221.
- ↑ Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA (January 2001). "Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia". Circulation. 103 (2): 196–200. PMID 11208676.
- ↑ Behere SP, Weindling SN (2016-5). "Catecholaminergic polymorphic ventricular tachycardia: An exciting new era". Annals of Pediatric Cardiology. 9 (2): 137–46. doi:10.4103/0974-2069.180645. PMC 4867798. PMID 27212848. Check date values in:
|date=(help)
Further reading
- Receptor defects cause inherited disorder CPVT
- Denervation successfully treats catecholaminergic polymorphic ventricular tachycardia
- Screening relatives of sudden-death victims provides likely cause of death and potentially saves lives
- Nakajima T, Kaneko Y, Taniguchi Y, Hayashi K, Takizawa T, Suzuki T, Nagai R (March 1997). "The mechanism of catecholaminergic polymorphic ventricular tachycardia may be triggered activity due to delayed afterdepolarization". European Heart Journal. 18 (3): 530–1. doi:10.1093/oxfordjournals.eurheartj.a015281. PMID 9076398.
- Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) Information sheet - Auckland District Health Board's Cardiac Inherited Disease Registry
- Clinical Data's PGxHealth Division Launches CPVT Cardiac Channelopathy Test - Business Wire
- SADS UK - What is CPVT
- Arrhythmogenesis in CPVT: Lessons Learned from a CPVT Mouse Model
External links
| Classification | |
|---|---|
| External resources |