Afterload
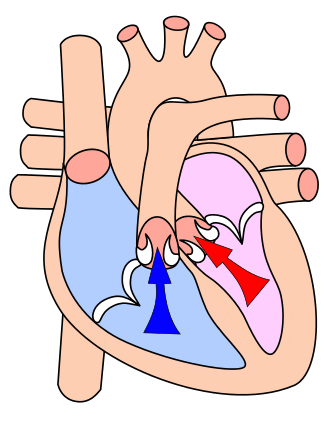
Afterload is the pressure against which the heart must work to eject blood during systole. In other words, it is the end load against which the heart contracts to eject blood. Afterload is readily broken into components: one factor is the aortic pressure the left ventricular muscle must overcome to eject blood. The greater the aortic/pulmonary pressure, the greater the after load on the left/right ventricle, respectively. Following Laplace's law, the tension upon the muscle fibers in the heart wall is the pressure within the ventricle multiplied by the volume within the ventricle divided by the wall thickness (this ratio is the other factor in setting the afterload). Therefore, when comparing a normal heart to a heart with a dilated left ventricle, if the aortic pressure is the same in both hearts, the dilated heart must create a greater tension to overcome the same aortic pressure to eject blood because it has a larger internal radius and volume. Thus, the dilated heart has a greater total load (tension) on the myocytes, i.e., has a higher afterload. This is also true in the eccentric hypertrophy consequent to high intensity aerobic training. Conversely, a concentrically hypertrophied left ventricle may have a lower afterload for a given aortic pressure. When contractility becomes impaired and the ventricle dilates, the afterload rises and limits output. This may start a vicious circle, in which cardiac output is reduced as oxygen requirements are increased.[1]
Afterload can also be described as the pressure that the chambers of the heart must generate in order to eject blood out of the heart and thus is a consequence of the aortic pressure (for the left ventricle) and pulmonic pressure or pulmonary artery pressure (for the right ventricle). The pressure in the ventricles must be greater than the systemic and pulmonary pressure to open the aortic and pulmonic valves, respectively. As afterload increases, cardiac output decreases. Cardiac imaging is a somewhat limited modality in defining afterload because it depends on the interpretation of volumetric data.
Pathology
Disease processes pathology that include indicators such as an increasing left ventricular afterload include elevated blood pressure and aortic valve disease.
Systemic hypertension (HTN) (elevated blood pressure) increases the left ventricular (LV) afterload because the LV must work harder to eject blood into the aorta. This is because the aortic valve won't open until the pressure generated in the left ventricle is higher than the elevated blood pressure in the aorta.[2]
Pulmonary hypertension (PH) is increased blood pressure within the right heart leading to the lungs. PH indicates a regionally applied increase in afterload dedicated to the right side of the heart, divided and isolated from the left heart by the interventricular septum.
In the natural aging process, aortic stenosis often increases afterload because the left ventricle must overcome the pressure gradient caused by the calcified and stenotic aortic valve in addition to the blood pressure in order to eject blood into the aorta. For instance, if the blood pressure is 120/80, and the aortic valve stenosis creates a trans-valvular gradient of 30 mmHg, the left ventricle has to generate a pressure of 110 mmHg in order to open the aortic valve and eject blood into the aorta.
Due to the increased afterload, the ventricle has to work harder to accomplish its goal of ejecting blood into the aorta. Thus in the long-term, the increased afterload (due to the stenosis) will result in hypertrophy of the left ventricle to account for the increased work required.
Aortic insufficiency (Aortic Regurgitation) increases afterload because a percentage of the blood that is ejected forward regurgitates back through the diseased aortic valve. This leads to elevated systolic blood pressure. The diastolic blood pressure in the aorta would fall, due to regurgitation. This would result in an increase in pulse pressure.
Mitral regurgitation (MR) decreases afterload. In ventricular systole under MR, regurgitant blood flows backwards/retrograde back and forth through a diseased and leaking mitral valve. The remaining blood loaded into the LV is then optimally ejected out through the aortic valve. With an extra pathway for blood flow through the mitral valve, the left ventricle does not have to work as hard to eject its blood, i.e. there is a decreased afterload.[3] Afterload is largely dependent upon aortic pressure.
See also
References
- ↑ Kasper, Dennis L; Braunwald, Eugene; Fauci, Anthony; et al. (2005). Harrison's Principles of Internal Medicine (16th ed.). New York: McGraw-Hill. p. 1346. ISBN 0-07-139140-1.
- ↑ Homoud, MK (Spring 2008). "Introduction to Cardiovascular Pathophysiology" (PDF). Tufts Open Courseware. Tufts University. p. 10. Retrieved 2010-05-04.
- ↑ Klabunde RE (2007-04-05). "Mitral Regurgitation". Cardiovascular Physiology Concepts. Richard E. Klabunde. Archived from the original on 3 January 2010. Retrieved 2010-01-01.
Further reading
- Ross, John (1976). "Afterload mismatch and preload reserve: A conceptual framework for the analysis of ventricular function". Progress in Cardiovascular Diseases. 18 (4): 255–64. doi:10.1016/0033-0620(76)90021-9. PMID 128034.
- Kasper, W.; Konstantinides, S.; Geibel, A.; Tiede, N.; Krause, T.; Just, H. (1997). "Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism". Heart. 77 (4): 346–9. doi:10.1136/hrt.77.4.346. PMC 484729. PMID 9155614.
- Mahler, Felix; Ross, John; O'Rourke, Robert A.; Covell, James W. (1975). "Effects of changes in preload, afterload and inotropic state on ejection and isovolumic phase measures of contractility in the conscious dog". The American Journal of Cardiology. 35 (5): 626–34. doi:10.1016/0002-9149(75)90048-X. PMID 1124716.
- Hachicha, Z.; Dumesnil, J. G.; Bogaty, P.; Pibarot, P. (2007). "Paradoxical Low-Flow, Low-Gradient Severe Aortic Stenosis Despite Preserved Ejection Fraction is Associated with Higher Afterload and Reduced Survival". Circulation. 115 (22): 2856–64. doi:10.1161/CIRCULATIONAHA.106.668681. PMID 17533183.
- Kelly, R. P.; Gibbs, H. H.; O'Rourke, M. F.; Daley, J. E.; Mang, K; Morgan, J. J.; Avolio, A. P. (1990). "Nitroglycerin has more favourable effects on left ventricular afterload than apparent from measurement of pressure in a peripheral artery". European Heart Journal. 11 (2): 138–44. doi:10.1093/oxfordjournals.eurheartj.a059669. PMID 2107077.