Daridorexant
Daridorexant (developmental code name ACT-541468), formerly known as nemorexant, is a dual orexin receptor antagonist (DORA) which was originated by Actelion Pharmaceuticals and is under development by Idorsia Pharmaceuticals for the treatment of insomnia.[1][2] It acts as a selective dual antagonist of the orexin receptors OX1 and OX2.[1][2] As of April 2020, daridorexant has passed its first phase III clinical trial for the treatment of insomnia.[1]
 | |
| Clinical data | |
|---|---|
| Other names | Nemorexant; ACT-541468 |
| Routes of administration | By mouth |
| Drug class | Orexin antagonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| PDB ligand | |
| Chemical and physical data | |
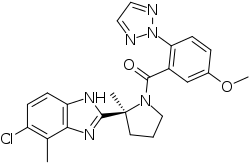
| Formula | C23H23ClN6O2 |
| Molar mass | 450.93 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- "Daridorexant - Idorsia Pharmaceuticals - AdisInsight".
- Equihua-Benítez AC, Guzmán-Vásquez K, Drucker-Colín R (July 2017). "Understanding sleep-wake mechanisms and drug discovery". Expert Opin Drug Discov. 12 (7): 643–657. doi:10.1080/17460441.2017.1329818. PMID 28511597.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.