Parthenolide
Parthenolide is a sesquiterpene lactone of the germacranolide class which occurs naturally in the plant feverfew (Tanacetum parthenium), after which it is named. It is found in highest concentration in the flowers and fruit. Parthenolide's molecular structure depiction is often incorrect regarding the stereochemistry of the epoxide, although X-ray single crystal structures are available.[1][2] The structure depicted here is the correct one.[3]
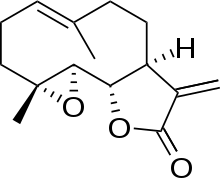 | |
| Names | |
|---|---|
| IUPAC name
(1aR,4E,7aS,10aS,10bR)-2,3,6,7,7a,8,10a,10b-octahydro-1a,5-dimethyl-8-methylene-oxireno[9,10]cyclodeca[1,2-b]furan-9(1aH)-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.220.558 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H20O3 | |
| Molar mass | 248.322 g·mol−1 |
| Melting point | 113 to 115 °C (235 to 239 °F; 386 to 388 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
Lack of solubility in water and bioavailability limits the potential of parthenolide as a drug, however synthetic analogs may be developed that could be absorbed to a more useful extent. The feverfew plant is used in herbalism for a variety of ailments. Some vendors specify the content of parthenolide in their products, believing it to be the primary constituent responsible for its activity.
In vitro biological activities
Parthenolide has a variety of reported in vitro biological activities, including:
- Inhibition HDAC1 protein without affecting other class I/II HDACs, which leads to sustained DNA damage response in certain cells (required for apoptosis).[4]
- Modulation of the NF-κB-mediated inflammatory responses in experimental atherosclerosis.[5]
- Promotion of axon regeneration in the peripheral nervous system with intraneural or systemic application.[6]
- Inducing apoptosis in acute myelogenous leukemia (AML) cells, leaving normal bone marrow cells relatively unscathed. Moreover, the compound may get at the root of the disease because it also kills stem cells that give rise to AML.[7]
- Activity against Leishmania amazonensis.[8]
- Microtubule-interfering activity.[9]
- Anti-inflammatory and anti-hyperalgesic effects.[10]
- Blocking lipopolysaccharide-induced osteolysis through the suppression of NF-κB activity.[11]
- Inducing apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells.[12]
- Inducing MITF M isoform downregulation and senescence in MITF-Mhigh melanoma cells[13]
- Agonist of the adiponectin receptor 2 (AdipoR2).[14]
- Inhibition of mammalian thioredoxin reductase [15]
References
- Quick, Andrew; Rogers, Donald (1976). "Crystal and molecular structure of parthenolide [4,5-epoxygermacra-1(10),11(13)-dien-12,6-olactone]". Journal of the Chemical Society, Perkin Transactions 2 (4): 465. doi:10.1039/p29760000465. ISSN 0300-9580.
- Long, Jing; Ding, Ya-Hui; Wang, Pan-Pan; Zhang, Quan; Chen, Yue (2013-10-18). "Protection-Group-Free Semisyntheses of Parthenolide and Its Cyclopropyl Analogue". The Journal of Organic Chemistry. 78 (20): 10512–10518. doi:10.1021/jo401606q. ISSN 0022-3263.
- Li, Xingjian; Payne, Daniel; Ampolu, Badarinath; Bland, Nicholas; Brown, Jane T; Dutton, Mark; Fitton, Catherine; Guliver, Abigail; Hale, Lee. "Derivatisation of Parthenolide to Address Chemoresistant Chronic Lymphocytic Leukaemia". doi:10.26434/chemrxiv.7629680. Cite journal requires
|journal=(help) - Rajendran P, Ho E, Williams DE, Dashwood RH (2011). "Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells". Clinical Epigenetics. 3 (1): 4. doi:10.1186/1868-7083-3-4. PMC 3255482. PMID 22247744.
- López-Franco O, Hernández-Vargas P, Ortiz-Muñoz G, Sanjuán G, Suzuki Y, Ortega L, Blanco J, Egido J, Gómez-Guerrero C (August 2006). "Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis". Arteriosclerosis, Thrombosis, and Vascular Biology. 26 (8): 1864–70. doi:10.1161/01.ATV.0000229659.94020.53. PMID 16741149.
- Gobrecht P, Andreadaki A, Diekmann H, Heskamp A, Leibinger M, Fischer D (April 2016). "Promotion of Functional Nerve Regeneration by Inhibition of Microtubule Detyrosination". The Journal of Neuroscience. 36 (14): 3890–902. doi:10.1523/JNEUROSCI.4486-15.2016. PMID 27053198.
- Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT (June 2005). "The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells". Blood. 105 (11): 4163–9. doi:10.1182/blood-2004-10-4135. PMC 1895029. PMID 15687234.
- Tiuman TS, Ueda-Nakamura T, Garcia Cortez DA, Dias Filho BP, Morgado-Díaz JA, de Souza W, Nakamura CV (January 2005). "Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium". Antimicrobial Agents and Chemotherapy. 49 (1): 176–82. doi:10.1128/AAC.49.11.176-182.2005. PMC 538891. PMID 15616293.
- Miglietta A, Bozzo F, Gabriel L, Bocca C (October 2004). "Microtubule-interfering activity of parthenolide". Chemico-Biological Interactions. 149 (2–3): 165–73. doi:10.1016/j.cbi.2004.07.005. PMID 15501437.
- Feltenstein MW, Schühly W, Warnick JE, Fischer NH, Sufka KJ (October 2004). "Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear's foot". Pharmacology Biochemistry and Behavior. 79 (2): 299–302. doi:10.1016/j.pbb.2004.08.008. PMID 15501305.
- Yip KH, Zheng MH, Feng HT, Steer JH, Joyce DA, Xu J (November 2004). "Sesquiterpene lactone parthenolide blocks lipopolysaccharide-induced osteolysis through the suppression of NF-kappaB activity". Journal of Bone and Mineral Research. 19 (11): 1905–16. doi:10.1359/JBMR.040919. PMID 15476591.
- Zunino SJ, Ducore JM, Storms DH (August 2007). "Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells". Cancer Letters. 254 (1): 119–27. doi:10.1016/j.canlet.2007.03.002. PMID 17470383.
- Hartman ML, Talar B, Sztiller-Sikorska M, Nejc D, Czyz M (February 2016). "Parthenolide induces MITF-M downregulation and senescence in patient-derived MITF-M(high) melanoma cell populations". Oncotarget. 7 (8): 9026–40. doi:10.18632/oncotarget.7030. PMC 4891023. PMID 26824319.
- Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, Zan W, Lin D, Zhao Y, Zhang Z (2013). "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLOS One. 8 (5): e63354. doi:10.1371/journal.pone.0063354. PMC 3653934. PMID 23691032.
- Duan D, Zhang J, Yao J, Liu Y, Fang J (May 2016). "Targeting Thioredoxin Reductase by Parthenolide Contributes to Inducing Apoptosis of HeLa Cells". The Journal of Biological Chemistry. 291 (19): 10021–31. doi:10.1074/jbc.M115.700591. PMC 4858956. PMID 27002142.
External links
- Guzman ML, Jordan CT (September 2005). "Feverfew: weeding out the root of leukaemia". Expert Opinion on Biological Therapy. 5 (9): 1147–52. doi:10.1517/14712598.5.9.1147. PMID 16120045.