Typhoid fever
| Typhoid fever | |
|---|---|
| Synonyms | Slow fever, typhoid |
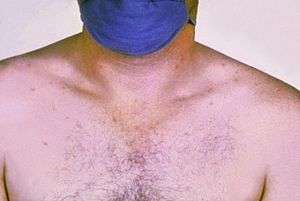 | |
| Rose spots on the chest of a person with typhoid fever | |
| Specialty | Infectious disease |
| Symptoms | Fever, abdominal pain, headache, rash[1] |
| Usual onset | 6–30 days after exposure[1][2] |
| Causes | Salmonella typhi (spread by food or water contaminated with feces)[3][4] |
| Risk factors | Poor sanitation, poor hygiene.[3] |
| Diagnostic method | Bacterial culture, DNA detection[2][3][5] |
| Differential diagnosis | Other infectious diseases[6] |
| Prevention | Typhoid vaccine, handwashing[2][7] |
| Treatment | Antibiotics[3] |
| Frequency | 12.5 million (2015)[8] |
| Deaths | 149,000 (2015)[9] |
Typhoid fever, also known simply as typhoid, is a bacterial infection due to Salmonella typhi that causes symptoms.[3] Symptoms may vary from mild to severe and usually begin six to thirty days after exposure.[1][2] Often there is a gradual onset of a high fever over several days;[1] weakness, abdominal pain, constipation, and headaches also commonly occur.[2][6] Diarrhea is uncommon and vomiting is not usually severe.[6] Some people develop a skin rash with rose colored spots.[2] In severe cases there may be confusion.[6] Without treatment, symptoms may last weeks or months.[2] Other people may carry the bacterium without being affected; however, they are still able to spread the disease to others.[4] Typhoid fever is a type of enteric fever, along with paratyphoid fever.[3]
The cause is the bacterium Salmonella Typhi, also known as Salmonella enterica serotype Typhi, growing in the intestines and blood.[2][6] Typhoid is spread by eating or drinking food or water contaminated with the feces of an infected person.[4] Risk factors include poor sanitation and poor hygiene.[3] Those who travel in the developing world are also at risk.[6] Only humans can be infected.[4] Symptoms are similar to those of many other infectious diseases.[6] Diagnosis is by either culturing the bacteria or detecting the bacterium's DNA in the blood, stool, or bone marrow.[2][3][5] Culturing the bacterium can be difficult.[10] Bone marrow testing is the most accurate.[5]
A typhoid vaccine can prevent about 40% to 90% of cases during the first two years.[7] The vaccine may have some effect for up to seven years.[3] It is recommended for those at high risk or people traveling to areas where the disease is common.[4] Other efforts to prevent the disease include providing clean drinking water, good sanitation, and handwashing.[2][4] Until it has been confirmed that an individual's infection is cleared, the individual should not prepare food for others.[2] The disease is treated with antibiotics such as azithromycin, fluoroquinolones or third generation cephalosporins.[3] Resistance to these antibiotics has been developing, which has made treatment of the disease more difficult.[3]
In 2015, there were 12.5 million new cases worldwide.[8] The disease is most common in India.[3] Children are most commonly affected.[3][4] Rates of disease decreased in the developed world in the 1940s as a result of improved sanitation and use of antibiotics to treat the disease.[4] Each year in the United States, about 400 cases are reported and it is estimated that the disease occurs in about 6,000 people.[6][11] In 2015, it resulted in about 149,000 deaths worldwide – down from 181,000 in 1990 (about 0.3% of the global total).[9][12] The risk of death may be as high as 20% without treatment.[4] With treatment, it is between 1 and 4%.[3][4] Typhus is a different disease.[13] However, the name typhoid means "resembling typhus" due to the similarity in symptoms.[14]
Signs and symptoms
Classically, the course of untreated typhoid fever is divided into four distinct stages, each lasting about a week. Over the course of these stages, the patient becomes exhausted and emaciated.[15]
- In the first week, the body temperature rises slowly, and fever fluctuations are seen with relative bradycardia (Faget sign), malaise, headache, and cough. A bloody nose (epistaxis) is seen in a quarter of cases, and abdominal pain is also possible. A decrease in the number of circulating white blood cells (leukopenia) occurs with eosinopenia and relative lymphocytosis; blood cultures are positive for Salmonella Typhi or S. paratyphi. The Widal test is usually negative in the first week.[16]
- In the second week, the person is often too tired to get up, with high fever in plateau around 40 °C (104 °F) and bradycardia (sphygmothermic dissociation or Faget sign), classically with a dicrotic pulse wave. Delirium is frequent, often calm, but sometimes agitated. This delirium gives to typhoid the nickname of "nervous fever". Rose spots appear on the lower chest and abdomen in around a third of patients. Rhonchi are heard in lung bases.
- The abdomen is distended and painful in the right lower quadrant, where borborygmi can be heard. Diarrhea can occur in this stage: six to eight stools in a day, green, comparable to pea soup, with a characteristic smell. However, constipation is also frequent. The spleen and liver are enlarged (hepatosplenomegaly) and tender, and liver transaminases are elevated. The Widal test is strongly positive, with antiO and antiH antibodies. Blood cultures are sometimes still positive at this stage.
- (The major symptom of this fever is that the fever usually rises in the afternoon up to the first and second week.)
- In the third week of typhoid fever, a number of complications can occur:
- Intestinal haemorrhage due to bleeding in congested Peyer's patches; this can be very serious, but is usually not fatal.
- Intestinal perforation in the distal ileum: this is a very serious complication and is frequently fatal. It may occur without alarming symptoms until septicaemia or diffuse peritonitis sets in.
- Encephalitis
- Respiratory diseases such as pneumonia and acute bronchitis
- Neuropsychiatric symptoms (described as "muttering delirium" or "coma vigil"), with picking at bedclothes or imaginary objects.
- Metastatic abscesses, cholecystitis, endocarditis, and osteitis
- The fever is still very high and oscillates very little over 24 hours. Dehydration ensues, and the patient is delirious (typhoid state). One-third of affected individuals develop a macular rash on the trunk.
- Platelet count goes down slowly and risk of bleeding rises.
- By the end of third week, the fever starts subsiding
Cause

Bacteria
The Gram-negative bacterium that causes typhoid fever is Salmonella Typhi, also known as Salmonella enterica serotype Typhi.[17] There are two main types of Typhi namely the ST1 and ST2 based on MLST subtyping scheme, which are currently widespread globally.[18]
Transmission
Salmonella Typhi is spread through the fecal-oral route from individuals that are currently infected and from asymptomatic carriers of the bacteria.[19] Unlike other strains of Salmonella, there are no animal carriers of S. Typhi.[19] Humans are the only known carriers of the bacteria.[19] An asymptomatic human carrier is an individual who is still excreting S. Typhi in their stool a year after the acute stage of the infection.[19] Human carriers are responsible for the transmission of the bacteria in endemic regions of the world.[19]
Diagnosis
Diagnosis is made by any blood, bone marrow or stool cultures and with the Widal test (demonstration of antibodies against Salmonella antigens O-somatic and H-flagellar). In epidemics and less wealthy countries, after excluding malaria, dysentery, or pneumonia, a therapeutic trial time with chloramphenicol is generally undertaken while awaiting the results of the Widal test and cultures of the blood and stool.[20]
The Widal test is time-consuming, and prone to significant false positive results. The test may be also falsely negative in the early course of illness. However, unlike Typhidot test Widal test quantifies the specimen with titres.
Typhidot is a medical test consisting of a dot ELISA kit that detects IgM and IgG antibodies against the outer membrane protein (OMP) of the Salmonella typhi. The typhidot test becomes positive within 2–3 days of infection and separately identifies IgM and IgG antibodies. The test is based on the presence of specific IgM and IgG antibodies to a specific 50Kd OMP antigen, which is impregnated on nitrocellulose strips. IgM shows recent infection whereas IgG signifies remote infection. The most important limitation of this test is that it is not quantitative and result is only positive or negative.
The term 'enteric fever' is a collective term that refers to severe typhoid and paratyphoid.[21]
Prevention
Sanitation and hygiene are important to prevent typhoid. Typhoid does not affect animals other than humans. Typhoid can only spread in environments where human feces are able to come into contact with food or drinking water. Careful food preparation and washing of hands are crucial to prevent typhoid. Industrialization, and in particular, the invention of the automobile, contributed greatly to the elimination of typhoid fever, as it eliminated the public health hazards associated with having horse manure in the public street which led to large number of flies.[22] According to statistics from the United States Centers for Disease Control and Prevention (CDC), the chlorination of drinking water has led to dramatic decreases in the transmission of typhoid fever in the United States.
Vaccination
Two typhoid vaccines are licensed for use for the prevention of typhoid:[7] the live, oral Ty21a vaccine (sold as Vivotif by Crucell Switzerland AG) and the injectable typhoid polysaccharide vaccine (sold as Typhim Vi by Sanofi Pasteur and 'Typherix by GlaxoSmithKline). Both are efficacious and recommended for travellers to areas where typhoid is endemic. Boosters are recommended every five years for the oral vaccine and every two years for the injectable form.[23] An older, killed-whole-cell vaccine is still used in countries where the newer preparations are not available, but this vaccine is no longer recommended for use because it has a higher rate of side effects (mainly pain and inflammation at the site of the injection).[24]
To help decrease rates of typhoid fever in developing nations, the World Health Organization (WHO) endorsed the use of a vaccination program starting in 1999. Vaccinations have proven to be a great way at controlling outbreaks in high incidence areas. Just as important, it is also very cost-effective. Vaccination prices are normally low, less than US $1 per dose. Because the price is low, poverty-stricken communities are more willing to take advantage of the vaccinations.[25] Although vaccination programs for typhoid have proven to be effective, they alone cannot eliminate typhoid fever.[25] Combining the use of vaccines along with increasing public health efforts is the only proven way to control this disease.[25]
Since the 1990s there have been two typhoid fever vaccines recommended by the World Health Organization. The ViPS vaccine is given via injection, while the Ty21a is taken through capsules. It is recommended only people 2 years or older be vaccinated with the ViPS vaccine and requires a revaccination after 2–3 years with a 55–72% vaccine efficacy. The alternative Ty21a vaccine is recommended for people 5 years or older, and has a 5-7-year duration with a 51–67% vaccine efficacy. The two different vaccines have been proven as a safe and effective treatment for epidemic disease control in multiple regions.[26]
A version combined with hepatitis A is also available.[27]
Treatment
The rediscovery of oral rehydration therapy in the 1960s provided a simple way to prevent many of the deaths of diarrheal diseases in general.
Where resistance is uncommon, the treatment of choice is a fluoroquinolone such as ciprofloxacin.[21][28] Otherwise, a third-generation cephalosporin such as ceftriaxone or cefotaxime is the first choice.[29][30][31][32] Cefixime is a suitable oral alternative.[33][34]
Typhoid fever, when properly treated, is not fatal in most cases. Antibiotics, such as ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole, amoxicillin, and ciprofloxacin, have been commonly used to treat typhoid fever in microbiology.[35] Treatment of the disease with antibiotics reduces the case-fatality rate to about 1%.[36]
Without treatment, some patients develop sustained fever, bradycardia, hepatosplenomegaly, abdominal symptoms and, occasionally, pneumonia. In white-skinned patients, pink spots, which fade on pressure, appear on the skin of the trunk in up to 20% of cases. In the third week, untreated cases may develop gastrointestinal and cerebral complications, which may prove fatal in up to 10–20% of cases. The highest case fatality rates are reported in children under 4 years. Around 2–5% of those who contract typhoid fever become chronic carriers, as bacteria persist in the biliary tract after symptoms have resolved.[37]
Surgery
Surgery is usually indicated in cases of intestinal perforation. Most surgeons prefer simple closure of the perforation with drainage of the peritoneum. Small-bowel resection is indicated for patients with multiple perforations.
If antibiotic treatment fails to eradicate the hepatobiliary carriage, the gallbladder should be resected. Cholecystectomy is not always successful in eradicating the carrier state because of persisting hepatic infection.
Resistance
As resistance to ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole, and streptomycin is now common, these agents have not been used as first–line treatment of typhoid fever for almost 20 years. Typhoid resistant to these agents is known as multidrug-resistant typhoid (MDR typhoid).
Ciprofloxacin resistance is an increasing problem, especially in the Indian subcontinent and Southeast Asia. Many centres are shifting from using ciprofloxacin as the first line for treating suspected typhoid originating in South America, India, Pakistan, Bangladesh, Thailand, or Vietnam. For these people, the recommended first-line treatment is ceftriaxone. Also, azithromycin has been suggested to be better at treating typhoid in resistant populations than both fluoroquinolone drugs and ceftriaxone.[28] Azithromycin significantly reduces relapse rates compared with ceftriaxone.
A separate problem exists with laboratory testing for reduced susceptibility to ciprofloxacin: current recommendations are that isolates should be tested simultaneously against ciprofloxacin (CIP) and against nalidixic acid (NAL), and that isolates that are sensitive to both CIP and NAL should be reported as "sensitive to ciprofloxacin", but that isolates testing sensitive to CIP but not to NAL should be reported as "reduced sensitivity to ciprofloxacin". However, an analysis of 271 isolates showed that around 18% of isolates with a reduced susceptibility to ciprofloxacin (MIC 0.125–1.0 mg/l) would not be picked up by this method.[38] How this problem can be solved is not certain, because most laboratories around the world (including the West) are dependent on disk testing and cannot test for MICs.
Epidemiology
In 2000, typhoid fever caused an estimated 21.7 million illnesses and 217,000 deaths.[39] It occurs most often in children and young adults between 5 and 19 years old.[40] In 2013 it resulted in about 161,000 deaths – down from 181,000 in 1990.[12] Infants, children, and adolescents in south-central and Southeast Asia experience the greatest burden of illness.[41] Outbreaks of typhoid fever are also frequently reported from sub-Saharan Africa and countries in Southeast Asia.[42][43][44] Historically, in the pre-antibiotic era, the case fatality rate of typhoid fever was 10–20%. Today, with prompt treatment, it is less than 1%.[45] However, about 3–5% of individuals who are infected will develop a chronic infection in the gall bladder.[46] Since S. Typhi is human-restricted, these chronic carriers become the crucial reservoir, which can persist for decades for further spread of the disease, further complicating the identification and treatment of the disease.[47] Lately, the study of Typhi associated with a large outbreak and a carrier at the genome level provides new insights into the pathogenesis of the pathogen.[48][49]
In industrialized nations, water sanitation and food handling improvements have reduced the number of cases.[50] Developing nations, such as those found in parts of Asia and Africa, have the highest rates of typhoid fever. These areas have a lack of access to clean water, proper sanitation systems, and proper health care facilities. For these areas, such access to basic public health needs is not in the near future.[51]
History

In 430 BC, a plague, which some believe to have been typhoid fever, killed one-third of the population of Athens, including their leader Pericles. Following this disaster, the balance of power shifted from Athens to Sparta, ending the Golden Age of Pericles that had marked Athenian dominance in the Greek ancient world. The ancient historian Thucydides also contracted the disease, but he survived to write about the plague. His writings are the primary source on this outbreak, and modern academics and medical scientists consider typhoid fever the most likely cause. In 2006, a study detected DNA sequences similar to those of the bacterium responsible for typhoid fever in dental pulp extracted from a burial pit dated to the time of the outbreak.[52]
The cause of the plague has long been disputed and other scientists have disputed the findings, citing serious methodologic flaws in the dental pulp-derived DNA study.[53] The disease is most commonly transmitted through poor hygiene habits and public sanitation conditions; during the period in question related to Athens above, the whole population of Attica was besieged within the Long Walls and lived in tents.
A pair of epidemics struck the Mexican highlands in 1545 and 1576, causing an estimated 7 to 17 million deaths.[54] A study published in 2018 suggests that the cause was typhoid fever.[55][56]
Some historians believe that the English colony of Jamestown, Virginia, died out from typhoid. Typhoid fever killed more than 6000 settlers in the New World between 1607 and 1624.[57]
A long-held belief is that 9th US President William Henry Harrison died of pneumonia, but recent studies suggest he likely died from typhoid. This disease may also have been a contributing factor in the death of 12th US President Zachary Taylor due to the unsanitary conditions in Washington, D.C., in the mid-19th century.[58][59]
During the American Civil War, 81,360 Union soldiers died of typhoid or dysentery, far more than died of battle wounds.[60] In the late 19th century, the typhoid fever mortality rate in Chicago averaged 65 per 100,000 people a year. The worst year was 1891, when the typhoid death rate was 174 per 100,000 people.[61]
During the Spanish–American War, American troops were exposed to typhoid fever in stateside training camps and overseas, largely due to inadequate sanitation systems. The Surgeon General of the Army, George Miller Sternberg, suggested that the War Department create a Typhoid Fever Board. Major Walter Reed, Edward O. Shakespeare, and Victor C. Vaughan were appointed August 18, 1898, with Reed being designated the President of the Board. The Typhoid Board determined that during the war, more soldiers died from this disease than from yellow fever or from battle wounds. The Board promoted sanitary measures including latrine policy, disinfection, camp relocation, and water sterilization, but by far the most successful antityphoid method was vaccination, which became compulsory in June 1911 for all federal troops.[62]

The most notorious carrier of typhoid fever, but by no means the most destructive, was Mary Mallon, also known as Typhoid Mary. In 1907, she became the first carrier in the United States to be identified and traced. She was a cook in New York who was closely associated with 53 cases and three deaths.[63] Public health authorities told Mary to give up working as a cook or have her gall bladder removed, as she had a chronic infection that kept her active as a carrier of the disease. Mary quit her job, but returned later under a false name. She was detained and quarantined after another typhoid outbreak. She died of pneumonia after 26 years in quarantine.
Development of vaccination
During the course of treatment of a typhoid outbreak in a local village in 1838, English country doctor William Budd realised the "poisons" involved in infectious diseases multiplied in the intestines of the sick, were present in their excretions, and could be transmitted to the healthy through their consumption of contaminated water.[64] He proposed strict isolation or quarantine as a method for containing such outbreaks in the future.[65] The medical and scientific communities did not identify the role of microorganisms in infectious disease until the work of Robert Koch and Louis Pasteur.

In 1880, Karl Joseph Eberth described a bacillus that he suspected was the cause of typhoid.[66][67][68] In 1884, pathologist Georg Theodor August Gaffky (1850–1918) confirmed Eberth's findings,[69] and the organism was given names such as Eberth's bacillus, Eberthella Typhi, and Gaffky-Eberth bacillus. Today, the bacillus that causes typhoid fever goes by the scientific name Salmonella enterica enterica, serovar Typhi.
The British bacteriologist Almroth Edward Wright first developed an effective typhoid vaccine at the Army Medical School in Netley, Hampshire. It was introduced in 1896 and used successfully by the British during the Boer War in South Africa.[70] At that time, typhoid often killed more soldiers at war than were lost due to enemy combat. Wright further developed his vaccine at a newly opened research department at St Mary's Hospital Medical School in London from 1902, where he established a method for measuring protective substances (opsonin) in human blood.
Citing the example of the Second Boer War, during which many soldiers died from easily preventable diseases, Wright convinced the British Army that 10 million vaccine doses should be produced for the troops being sent to the Western Front, thereby saving up to half a million lives during World War I.[71] The British Army was the only combatant at the outbreak of the war to have its troops fully immunized against the bacterium. For the first time, their casualties due to combat exceeded those from disease.[72]
In 1909, Frederick F. Russell, a U.S. Army physician, adopted Wright's typhoid vaccine for use with the US Army, and two years later, his vaccination program became the first in which an entire army was immunized. It eliminated typhoid as a significant cause of morbidity and mortality in the U.S. military.[73]
Most developed countries saw declining rates of typhoid fever throughout the first half of the 20th century due to vaccinations and advances in public sanitation and hygiene. In 1908, the chlorination of public drinking water was a significant step in the US in the control of typhoid fever. The first permanent disinfection of drinking water in the U.S. was made to the Jersey City, New Jersey, water supply. Credit for the decision to build the chlorination system has been given to John L. Leal.[74] The chlorination facility was designed by George W. Fuller.[75] In 1942 doctors introduced antibiotics in clinical practice, greatly reducing mortality. Today, the incidence of typhoid fever in developed countries is around five cases per million people per year.
A notable outbreak occurred in Aberdeen, Scotland, in 1964. This was due to contaminated tinned meat sold at the city's branch of the William Low chain of stores. No fatalities resulted.
In 2004–05 an outbreak in the Democratic Republic of Congo resulted in more than 42,000 cases and 214 deaths.[40]
Names
The disease has been referred to by various names, often associated with symptoms, such as gastric fever, enteric fever, abdominal typhus, infantile remittant fever, slow fever, nervous fever, and pythogenic fever.
Notable cases
- William Henry Harrison, the 9th President of the United States of America, died 32 days into his term, in 1841. This is the shortest term served by a United States President.
- Edward VII of England, while still Prince of Wales, had a near fatal case of typhoid fever in 1871.[76] It was thought at the time that his father, the Prince Consort Albert, had also died of typhoid fever (in 1861)[77] but this is disputed.
- Gerard Manley Hopkins, English poet, died of typhoid fever in 1889.[78]
- Dr HJH 'Tup' Scott, captain of the 1886 Australian cricket team that toured England, died of typhoid in 1910.[79]
- Arnold Bennett, English novelist, died in 1932 of typhoid, two months after drinking a glass of water in a Paris hotel to prove it was safe.[80]
- Hakaru Hashimoto, Japanese medical scientist, died of typhoid fever in 1934.[81]
- Heath Bell, a relief pitcher for the San Diego Padres, acquired typhoid on a 2010 trip to Fiji, and survived.[82]
- Lourdes Van-Dúnem, Angolan singer, died in 2006.[83]
- William Wallace Lincoln, the son of former US president Abraham and Mary Todd Lincoln, died of typhoid in 1862.[84]
- Leland Stanford Jr., son of American tycoon and politician A. Leland Stanford and eponym of Leland Stanford Junior University, died of typhoid fever in 1884 at the age of 15.[85]
- Lizzie van Zyl, South African child inmate of the Bloemfontein concentration camp during the Second Boer War, died of typhoid fever in 1901.
See also
References
- 1 2 3 4 Anna E. Newton (2014). "3 Infectious Diseases Related To Travel". CDC health information for international travel 2014 : the yellow book. ISBN 9780199948499. Archived from the original on 2015-07-02.
- 1 2 3 4 5 6 7 8 9 10 11 "Typhoid Fever". cdc.gov. May 14, 2013. Archived from the original on 6 June 2016. Retrieved 28 March 2015.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Wain, J; Hendriksen, RS; Mikoleit, ML; Keddy, KH; Ochiai, RL (21 March 2015). "Typhoid fever". Lancet. 385 (9973): 1136–45. doi:10.1016/s0140-6736(13)62708-7. PMID 25458731.
- 1 2 3 4 5 6 7 8 9 10 "Typhoid vaccines: WHO position paper" (PDF). Wkly Epidemiol Rec. 83 (6): 49–59. February 8, 2008. PMID 18260212. Archived (PDF) from the original on April 2, 2015.
- 1 2 3 Crump, JA; Mintz, ED (15 January 2010). "Global trends in typhoid and paratyphoid Fever". Clin Infect Dis. 50 (2): 241–6. doi:10.1086/649541. PMC 2798017. PMID 20014951.
- 1 2 3 4 5 6 7 8 "Typhoid Fever". cdc.gov. May 14, 2013. Archived from the original on 2 April 2015. Retrieved 28 March 2015.
- 1 2 3 Milligan, R; Paul, M; Richardson, M; Neuberger, A (31 May 2018). "Vaccines for preventing typhoid fever". The Cochrane Database of Systematic Reviews. 5: CD001261. doi:10.1002/14651858.CD001261.pub4. PMID 29851031.
- 1 2 GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- 1 2 GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ↑ Alan J. Magill (2013). Hunter's tropical medicine and emerging infectious diseases (9th ed.). London: Saunders/Elsevier. pp. 568–572. ISBN 9781455740437. Archived from the original on 2017-02-28.
- ↑ Jackson, BR; Iqbal, S; Mahon, B (27 March 2015). "Updated Recommendations for the Use of Typhoid Vaccine – Advisory Committee on Immunization Practices, United States, 2015". Morb Mortal Wkly Rep. 64 (11): 305–308. PMC 4584884. PMID 25811680.
- 1 2 GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013". Lancet. 385 (9963): 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ↑ Cunha BA (March 2004). "Osler on typhoid fever: differentiating typhoid from typhus and malaria". Infect Dis Clin N Am. 18 (1): 111–25. doi:10.1016/S0891-5520(03)00094-1. PMID 15081508.
- ↑ "Oxford English Dictionary (Online)". p. typhoid, adj. and n. Archived from the original on 11 January 2008. Retrieved 28 March 2015.
Resembling or characteristic of typhus
- ↑ "Typhoid". Merriam Webster Dictionary. Archived from the original on 2013-07-02. Retrieved 2013-06-24.
- ↑ Kumar P, Kumar R (2016). "Enteric Fever". Indian J Pediatr. 84 (3): 227–230. doi:10.1007/s12098-016-2246-4. PMID 27796818.
- ↑ "Typhoid Fever Information for Health Professionals". CDC. May 14, 2013. Archived from the original on 20 August 2016. Retrieved 20 August 2016.
- ↑ Yap, Kien-Pong; et al. (2016). "Global MLST of Salmonella Typhi Revisited in Post-genomic Era: Genetic Conservation, Population Structure, and Comparative Genomics of Rare Sequence Types". Front Microbiol. 7: 270. doi:10.3389/fmicb.2016.00270. PMC 4774407. PMID 26973639.
- 1 2 3 4 5 Eng, SK; Pusparajah, P; Mutalib, NS; Ser, HL; Chan, KG; Lee, LH (9 June 2015). "Salmonella:A review on pathogenesis, epidemiology and antibiotic resistance". Taylor & Francis Online. 8 (3): 284–293. doi:10.1080/21553769.2015.1051243.
- ↑ Ryan KJ; Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- 1 2 Parry CM, Beeching NJ (2009). "Treatment of enteric fever". Br Med J. 338: b1159. doi:10.1136/bmj.b1159. PMID 19493937.
- ↑ "The Great Horse Manure Crisis of 1894". Archived from the original on 2015-05-25.
- ↑ Anwar, E; Goldberg, E; Fraser, A; Acosta, CJ; Paul, M; Leibovici, L (2 January 2014). "Vaccines for preventing typhoid fever". Cochrane Database Syst Rev (1): CD001261. doi:10.1002/14651858.CD001261.pub3. PMID 24385413.
- ↑ Marathe, Sandhya A.; Lahiri, Amit; Negi, Vidya Devi; Chakravortty, Dipshikha (2012). "Typhoid fever & vaccine development: a partially answered question". Indian J Med Res. 135 (2): 161–169. ISSN 0971-5916. PMC 3336846. PMID 22446857.
- 1 2 3 Date, Kashmira A.; Bentsi-Enchill, Adwoa; Marks, Florian; Fox, Kimberley (2015-06-19). "Typhoid fever vaccination strategies". Vaccine. 33: C55–C61. doi:10.1016/j.vaccine.2015.04.028. PMID 25902360. Retrieved 2016-12-15.
- ↑ Date, Kashmira A.; Bentsi-Enchill, Adwoa; Marks, Florian; Fox, Kimberley (June 2015). "Typhoid fever vaccination strategies". Vaccine. 33: C55–C61. doi:10.1016/j.vaccine.2015.04.028. PMID 25902360.
- ↑ health, NPS: Better choices, Better. "Vivaxim Solution for injection". NPS MedicineWise. Archived from the original on 1 October 2015. Retrieved 10 April 2017.
- 1 2 Effa EE, Lassi ZS, Critchley JA, Garner P, Sinclair D, Olliaro PL, Bhutta ZA (2011). Bhutta, Zulfiqar A, ed. "Fluoroquinolones for treating typhoid and paratyphoid fever (enteric fever)". Cochrane Database Syst Rev (10): CD004530. doi:10.1002/14651858.CD004530.pub4. PMID 21975746.
- ↑ Soe GB, Overturf GD (1987). "Treatment of typhoid fever and other systemic salmonelloses with cefotaxime, ceftriaxone, cefoperazone, and other newer cephalosporins". Rev Infect Dis. 9 (4): 719–36. doi:10.1093/clinids/9.4.719. JSTOR 4454162. PMID 3125577.
- ↑ Wallace MR, Yousif AA, Mahroos GA, Mapes T, Threlfall EJ, Rowe B, Hyams KC (1993). "Ciprofloxacin versus ceftriaxone in the treatment of multiresistant typhoid fever" (Submitted manuscript). Eur J Clin Microbiol Infect Dis. 12 (12): 907–910. doi:10.1007/BF01992163. PMID 8187784.
- ↑ Dutta P, Mitra U, Dutta S, De A, Chatterjee MK, Bhattacharya SK (2001). "Ceftriaxone therapy in ciprofloxacin treatment failure typhoid fever in children". Indian J Med Res. 113: 210–3. PMID 11816954.
- ↑ Коваленко А.Н.; et al. (2011). "Особенности клиники, диагностики и лечения брюшного тифа у лиц молодого возраста". Voen.-meditsinskii Zhurnal. 332 (1): 33–39.
- ↑ Bhutta ZA, Khan IA, Molla AM (1994). "Therapy of multidrug-resistant typhoid fever with oral cefixime vs. intravenous ceftriaxone". Pediatr Infect Dis J. 13 (11): 990–993. doi:10.1097/00006454-199411000-00010. PMID 7845753.
- ↑ Cao XT, Kneen R, Nguyen TA, Truong DL, White NJ, Parry CM (1999). "A comparative study of ofloxacin and cefixime for treatment of typhoid fever in children. The Dong Nai Pediatric Center Typhoid Study Group". Pediatr Infect Dis J. 18 (3): 245–8. PMID 10093945.
- ↑ Baron S et al.
- ↑ "Diarrhoeal Diseases (Updated February 2009)". Archived from the original on November 2, 2011. Retrieved 2013-04-25. . World Health Organization
- ↑ "WHO | Typhoid fever". www.who.int. Archived from the original on 2017-07-27. Retrieved 2017-08-10.
- ↑ Cooke FJ, Wain J, Threlfall EJ (2006). "Fluoroquinolone resistance in Salmonella Typhi (letter)". Br Med J. 333 (7563): 353–354. doi:10.1136/bmj.333.7563.353-b. PMC 1539082. PMID 16902221.
- ↑ Crump JA, Mintz ED (2010). "Global trends in typhoid and paratyphoid fever". Clin Infect Dis. 50 (2): 241–246. doi:10.1086/649541. PMC 2798017. PMID 20014951.
- 1 2 "Typhoid Fever". World Health Organization. Archived from the original on 2011-11-02. Retrieved 2007-08-28.
- ↑ Crump JA, Luby SP, Mintz ED (2004). "The global burden of typhoid fever". Bull World Health Organ. 82: 346–353.
- ↑ Muyembe-Tamfum JJ, Veyi J, Kaswa M, Lunguya O, Verhaegen J, Boelaert M (2009). "An outbreak of peritonitis caused by multidrug-resistant Salmonella Typhi in Kinshasa, Democratic Republic of Congo". Travel Med Infect Dis. 7 (40): 40–43. doi:10.1016/j.tmaid.2008.12.006. PMID 19174300.
- ↑ Baddam R, Kumar N, Thong KL, Ngoi ST, Teh CS, Yap KP, Chai LC, Avasthi TS, Ahmed N (2012). "Genetic fine structure of a Salmonella enterica serovar Typhi strain associated with the 2005 outbreak of typhoid fever in Kelantan, Malaysia". J Bacteriol. 194 (13): 3565–3566. doi:10.1128/jb.00581-12. PMC 3434757. PMID 22689247.
- ↑ Yap, Kien-Pong; et al. (2012). "Insights from the genome sequence of a Salmonella enterica serovar Typhi strain associated with a sporadic case of typhoid fever in Malaysia". J Bacteriol. 194 (18): 5124–5125. doi:10.1128/jb.01062-12. PMC 3430317. PMID 22933756.
- ↑ Heymann, David L., ed. (2008), Control of Communicable Diseases Manual, Washington, D.C.: American Public Health Association, pg 665. ISBN 978-0-87553-189-2.
- ↑ Levine; et al. (1982). "Precise estimation of the numbers of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area". J Infect Dis. 146 (6): 724–726. doi:10.1093/infdis/146.6.724.
- ↑ G Gonzalez-Escobedo; JM Marshall; JS Gunn (2010). "Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state". Nat Rev Microbiol. 9 (1): 9–14. doi:10.1038/nrmicro2490. PMC 3255095. PMID 21113180.
- ↑ Yap, Kien-Pong; et al. (2012). "Genome sequence and comparative pathogenomics analysis of a Salmonella enterica serovar Typhi strain associated with a typhoid carrier in Malaysia". J Bacteriol. 194 (21): 5970–5971. doi:10.1128/jb.01416-12. PMC 3486090. PMID 23045488.
- ↑ Yap, Kien-Pong; et al. (2014). "Comparative genomics of closely related Salmonella enterica serovar Typhi strains reveals genome dynamics and the acquisition of novel pathogenic elements". BMC Genomics. 15 (1): 1007. doi:10.1186/1471-2164-15-1007. PMC 4289253. PMID 25412680.
- ↑ Crump, John A.; Sjölund-Karlsson, Maria; Gordon, Melita A.; Parry, Christopher M. (2016-12-15). "Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections". Clin Microbiol Rev. 28 (4): 901–937. doi:10.1128/CMR.00002-15. ISSN 0893-8512. PMC 4503790. PMID 26180063.
- ↑ Khan, M. Imran; Pach 3rd, Khan; Khan, Ghulam Mustafa; Bajracharya, Deepak; Sahastrabuddhe, Sushant; Bhutta, Waqaas; Tahir, Rehman; Soofi, Sajid; Thapa, Chandra B. (2015-06-19). "Typhoid vaccine introduction: An evidence-based pilot implementation project in Nepal and Pakistan". Vaccine. 33: C62–C67. doi:10.1016/j.vaccine.2015.03.087. PMID 25937612. Retrieved 2016-12-15.
- ↑ Papagrigorakis MJ, Yapijakis C, Synodinos PN, Baziotopoulou-Valavani E (2006). "DNA examination of ancient dental pulp incriminates typhoid fever as a probable cause of the Plague of Athens". Int J Infect Dis. 10 (3): 206–214. doi:10.1016/j.ijid.2005.09.001. PMID 16412683.
- ↑ Shapiro B, Rambaut A, Gilbert MT (2006). "No proof that typhoid caused the Plague of Athens (a reply to Papagrigorakis et al.)". Int J Infect Dis. 10 (4): 334–335. doi:10.1016/j.ijid.2006.02.006. PMID 16730469.
- ↑ Acuna-Soto, R; Stahle, DW; Cleaveland, MK; Therrell, MD (April 2002). "Megadrought and megadeath in 16th century Mexico". Emerging Infectious Diseases. 8 (4): 360–2. doi:10.3201/eid0804.010175. PMC 2730237. PMID 11971767.
- ↑ Hersher, Rebecca (2018-01-15). "Salmonella May Have Caused Massive Aztec Epidemic, Study Finds". NRP. Retrieved 2018-01-15.
- ↑ Vågene, Åshild J.; Herbig, Alexander; Campana, Michael G.; Robles García, Nelly M.; Warinner, Christina; Sabin, Susanna; Spyrou, Maria A.; Andrades Valtueña, Aida; Huson, Daniel; Tuross, Noreen; Bos, Kirsten I.; Krause, Johannes (2018-01-15). "Salmonella enterica genomes from victims of a major sixteenth-century epidemic in Mexico". Nature Ecology & Evolution. 2 (3): 520–528. doi:10.1038/s41559-017-0446-6. PMID 29335577.
- ↑ Byrne, Joseph Patrick (2008). Encyclopedia of Pestilence, Pandemics, and Plagues: A-M. ABC-CLIO. p. 190. ISBN 978-0-313-34102-1. Archived from the original on 2014-01-03.
- ↑ McHugh, Jane; Mackowiak, Philip A. (2014-10-01). "Death in the White House: President William Henry Harrison's Atypical Pneumonia". Clin Infect Dis. 59 (7): 990–995. doi:10.1093/cid/ciu470. ISSN 1058-4838. PMID 24962997.
- ↑ Mchugh, Jane; Mackowiak, Philip A. (2014-03-31). "What Really Killed William Henry Harrison?". The New York Times. ISSN 0362-4331. Archived from the original on 2017-01-20. Retrieved 2017-01-19.
- ↑ Armies of Pestilence: The Effects of Pandemics on History Archived 2016-04-28 at the Wayback Machine.. James Clarke & Co. (2004). p.191. ISBN 0-227-17240-X
- ↑ "1900 Flow of Chicago River Reversed". Chicago Timeline. Chicago Public Library. Archived from the original on 2007-03-07. Retrieved 2007-02-08.
- ↑ Walter Reed Typhoid Fever, 1897–1911, "Archived copy". Archived from the original on 2014-10-06. Retrieved 2014-09-03. , Claude Moore Health Sciences Library. University of Virginia.
- ↑ "Nova: The Most Dangerous Woman in America". Archived from the original on 2010-04-26.
- ↑ Asimov, Asimov's Biographical Encyclopedia of Science and Technology 2nd Revised edition
- ↑ Aronson SM (1995). "William Budd and typhoid fever". R I Med. 78 (11): 310. PMID 8547718.
- ↑ C. J. Eberth (1880) "Die Organismen in den Organen bei Typhus abdominalis" (Organisms in the [internal] organs in cases of Typhus abdominalis), Archiv für pathologische Anatomie und Physiologie, 81 : 58–74.
- ↑ C. J. Eberth (1881) "Neue Untersuchungen über den Bacillus des Abdominaltyphus" (New investigations into the bacilli of abdominal typhoid), Archiv für pathologische Anatomie und Physiologie, 83 : 486–501.
- ↑ Eberth's findings were verified by Robert Koch: Koch, Robert (1881) "Zur Untersuchung von pathogenen Organismen" Archived 2017-04-23 at the Wayback Machine. (On the investigation of pathogenic organisms), Mitteilungen aus dem Kaiserlichen Gesundheitsamte, 1 : 1–49 ; see p. 45. Archived 2017-04-23 at the Wayback Machine.
- ↑ Gaffky (1884) "Zur Aetiology des Abdominaltyphus" Archived 2017-04-23 at the Wayback Machine. (On the etiology of abdominal typhus), Mittheilungen aus dem Kaiserlichen Gesundheitsamte, 2 : 372–420.
- ↑ "Sir Almroth Edward Wright". Encyclopædia Britannica. Archived from the original on 2013-11-11.
- ↑ "Library and Archive Catalogue". Royal Society. Retrieved 1 November 2010.
- ↑ "Medical lessons from World War I underscore need to keep developing antimicrobial drugs". MinnPost. Archived from the original on 30 January 2016. Retrieved 8 September 2017.
- ↑ USAMRMC: 50 Years of Dedication to the Warfighter 1958–2008 (PDF). U.S. Army Medical Research & Material Command (2008). 2008. p. 5. ASIN B003WYKJNY. Archived (PDF) from the original on 2013-02-14.
- ↑ Leal, John L. (1909). "The Sterilization Plant of the Jersey City Water Supply Company at Boonton, N.J." Proceedings American Water Works Association. pp. 100–9.
- ↑ Fuller, George W. (1909). "Description of the Process and Plant of the Jersey City Water Supply Company for the Sterilization of the Water of the Boonton Reservoir." Proceedings American Water Works Association. 110-34.
- ↑ Matthew, H. C. G. (September 2004; online edition May 2006) "Edward VII (1841–1910)" Archived 2016-03-02 at the Wayback Machine., Oxford Dictionary of National Biography, Oxford University Press, doi:10.1093/ref:odnb/32975, retrieved 24 June 2009 (subscription or UK public library membership required)
- ↑ Paulley, J.W. (1993). "The death of Albert Prince Consort: the case against typhoid fever". QJM. 86 (12): 837–841. doi:10.1093/oxfordjournals.qjmed.a068768. PMID 8108541. Archived from the original on 2015-12-08.
- ↑ Ruggles, Eleanor (1944) Gerard Manley Hopkins: A Life. Norton.
- ↑ Scott, Belinda F.. (1910-09-23) Biography – Henry James Herbert Scott – Australian Dictionary of Biography Archived 2011-02-21 at the Wayback Machine.. Adbonline.anu.edu.au. Retrieved on 2014-05-12.
- ↑ "Straw for Silence". The Spectator. Vol. 203. F.C. Westley. 1959. ISSN 0038-6952. OCLC 1766325. Retrieved March 16, 2011.
- ↑ Hakaru Hashimoto#Biography
- ↑ "Heath Bell Recovering After Bout With Typhoid Fever on Vacation". AOL News. 2011. Archived from the original on 2 March 2013. Retrieved 17 October 2011.
- ↑ "Angolan singer Lourdes Van-Dunem dies of typhoid". 2006. Archived from the original on 4 March 2016. Retrieved 23 October 2015.
- ↑ Dennis, Brady; Dennis, Brady (2011-09-29). "Willie Lincoln's death: A private agony for a president facing a nation of pain". The Washington Post. ISSN 0190-8286. Archived from the original on 2017-04-01. Retrieved 2017-03-12.
- ↑ "A History of Stanford". Stanford University. Retrieved 4 July 2018.
Further reading
- Easmon C (2005-04-01). "Typhoid fever and paratyphoid fever". Travel Health. Retrieved 2008-10-05.
- Harrison NG. "Walter Reed and Typhoid Fever, 1897–1911". Univ of Virginia. Retrieved 2008-10-05.
- Nicolson, Stuart (2008-06-26). "Typhoid left city (Aberdeen) 'under siege'". BBC News. Retrieved 2008-10-05.
- O'Hara C (2006-01-26). "Typhoid Fever Led To The Fall Of Athens". Elsevier. Retrieved 2008-10-05.
External links
| Classification | |
|---|---|
| External resources |
| Typhoid fever | |
|---|---|
| Specialty |
Infectious disease |
| Wikimedia Commons has media related to Typhoid fever. |
| Wikimedia Commons has media related to Salmonella. |
