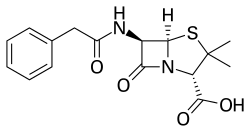
Benzylpenicillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Pfizerpen, other |
| Synonyms | penicillin G potassium,[1] penicillin G sodium |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a685013 |
| Pregnancy category | |
| Routes of administration | IV, IM |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 60 % |
| Metabolism | Liver |
| Elimination half-life | 30 min |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number |
E705 (antibiotics) |
| ECHA InfoCard |
100.000.461 |
| Chemical and physical data | |
| Formula | C16H18N2O4S |
| Molar mass | 334.4 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Benzylpenicillin, also known as penicillin G, is an antibiotic used to treat a number of bacterial infections.[2] This includes pneumonia, strep throat, syphilis, necrotizing enterocolitis, diphtheria, gas gangrene, leptospirosis, cellulitis, and tetanus.[2] It is not a first-line agent for pneumococcal meningitis.[2] Benzylpenicillin is given by injection into a vein or muscle.[1] Two long-acting forms benzathine benzylpenicillin and procaine benzylpenicillin are available for use by injection into a muscle.[2]
Side effects include diarrhea, seizures, and allergic reactions including anaphylaxis.[2] When used to treat syphilis a reaction known as Jarisch–Herxheimer may occur.[2] It is not recommended in those with a history of penicillin allergy.[2] Use during pregnancy is generally safe.[1] It is in the penicillin and β-lactam class of medications.[2]
Benzylpenicillin was discovered in 1929 by Alexander Fleming and came into commercial use in 1942.[3] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[4] The wholesale cost in the developing world is about 0.24 to 2.72 USD per day.[5] In the United States a course of treatment costs 100 to 200 USD.[6]
Medical uses
Antimicrobial potency
As an antibiotic, benzylpenicillin is noted to possess effectiveness mainly against Gram-positive organisms. Some Gram-negative organisms such as Neisseria gonorrhoeae and Leptospira weilii are also reported to be susceptible to benzylpenicillin.[7]
Adverse effects
Adverse effects can include hypersensitivity reactions including urticaria, fever, joint pains, rashes, angioedema, anaphylaxis, serum sickness-like reaction. Rarely CNS toxicity including convulsions (especially with high doses or in severe renal impairment), interstitial nephritis, haemolytic anaemia, leucopenia, thrombocytopenia, and coagulation disorders. Also reported diarrhoea (including antibiotic-associated colitis).
Benzylpenicillin serum concentrations can be monitored either by traditional microbiological assay or by more modern chromatographic techniques. Such measurements can be useful to avoid central nervous system toxicity in any person receiving large doses of the drug on a chronic basis, but they are especially relevant to patients with renal failure, who may accumulate the drug due to reduced urinary excretion rates.[8][9]
References
- 1 2 3 "Penicillin G Injection - FDA prescribing information, side effects and uses". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 10 December 2016.
- 1 2 3 4 5 6 7 8 WHO Model Formulary 2008 (PDF). World Health Organization. 2009. pp. 98, 105. ISBN 9789241547659. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ↑ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 490. ISBN 9783527607495. Archived from the original on 20 December 2016.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ↑ "Penicillin, Benzyl". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- ↑ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 95. ISBN 9781284057560.
- ↑ "Penicillin G" (PDF). Toku-E. 2010-10-10. Archived from the original (pdf) on 2016-03-03. Retrieved 2012-06-11.
- ↑ Fossieck B Jr, Parker RH. Neurotoxicity during intravenous infusion of penicillin. A review. J. Clin. Pharmacol. 14: 504- 512, 1974.
- ↑ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 1195-1196.