Colchicine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Colcrys, Mitigare, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682711 |
| Pregnancy category | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 45% |
| Protein binding | 35-44% |
| Metabolism | Metabolism, partly by CYP3A4 |
| Elimination half-life | 26.6-31.2 hours |
| Excretion | Faeces (65%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.000.544 |
| Chemical and physical data | |
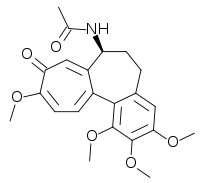
| Formula | C22H25NO6 |
| Molar mass | 399.437 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Colchicine (sold under brand names Colcrys or Mitigare) is a medication most commonly used to treat gout.[1][2][3] In addition to gout, colchicine is used to treat familial Mediterranean fever, pericarditis, and Behçet's disease.[2] Adverse effects are primarily gastrointestinal upset at high doses.[4]
It is a toxic alkaloid and secondary metabolite, originally extracted from plants of the genus Colchicum (autumn crocus, Colchicum autumnale, also known as "meadow saffron").[2][5][6]
Medical uses
Gout
Colchicine is an alternative for those unable to tolerate NSAIDs in gout.[7] At high doses, side effects (primarily gastrointestinal upset) limit its use.[2][8] At lower doses, it is well tolerated.[2][9][10][11] One review found low-quality evidence that low-dose colchicine (1.8 mg in one hour or 1.2 mg per day) reduced gout symptoms and pain, whereas high-dose colchicine (4.8 mg over 6 hours) was effective against pain, but caused more severe side effects, such as diarrhea, nausea or vomiting.[10]
For treating gout symptoms, colchicine is used orally with or without food, as symptoms first appear.[6] Subsequent doses may be needed if symptoms worsen.[6][10]
Other conditions
Colchicine is also used as an anti-inflammatory agent for long-term treatment of Behçet's disease.[12] It appears to have limited effect in relapsing polychondritis, as it may only be useful for the treatment of chondritis and mild skin symptoms.[13]
Colchicine is also used in addition to other therapy in the treatment of pericarditis.[12][14]
Colchicine is used widely in the treatment of familial Mediterranean fever,[12] in which it reduces attacks and the long-term risk of amyloidosis.[15]
Investigative uses
Colchicine has demonstrated efficacy for prevention of atrial fibrillation after cardiac surgery.[16]
Mechanism of action
In gout, inflammation in joints results from the precipitation of circulating uric acid, exceeding its solubility in blood and depositing as crystals of monosodium urate in and around synovial fluid and soft tissues of joints.[3] These crystal deposits cause inflammatory arthritis, which is initiated and sustained by mechanisms involving various proinflammatory mediators, such as cytokines.[3]
Under preliminary research are various mechanisms by which colchicine may interfere with gout inflammation:
- inhibits microtubule polymerization by binding to tubulin, one of the main constituents of microtubules[3]
- as availability of tubulin is essential to mitosis, colchicine may inhibit mitosis[3]
- inhibits activation and migration of neutrophils to sites of inflammation[6]
- interferes with the inflammasome complex found in neutrophils and monocytes that mediate interleukin-1β activation, a component of inflammation[6]
- inhibits superoxide anion production in response to urate crystals[3]
- interrupts mast cell degranulation[3]
Generally, colchicine appears to inhibit multiple proinflammatory mechanisms, while enabling increased levels of anti-inflammatory mediators.[3] Apart from inhibiting mitosis, colchicine inhibits neutrophil motility and activity, leading to a net anti-inflammatory effect, which has efficacy for inhibiting or preventing gout inflammation.[3][6]
Formulations and dosing
Trade names for colchicine are Colcrys or Mitigare which are manufactured as a dark– and light-blue capsule having a dose of 0.6 mg.[6][17] Colchicine is also prepared as a white, yellow, or purple pill (tablet) having a dose of 0.6 mg.[17]
Colchicine is typically prescribed to mitigate or prevent the onset of gout, or its continuing symptoms and pain, using a low-dose prescription of 0.6 to 1.2 mg per day, or a high-dose amount of up to 4.8 mg in the first 6 hours of a gout episode.[4][6][10] With an oral dose of 0.6 mg, peak blood levels occur within one to two hours.[5] For treating gout, the initial effects of colchicine occur in a window of 12 to 24 hours, with a peak within 48 to 72 hours.[6] It has a narrow therapeutic window, requiring monitoring of the subject for potential toxicity.[6] Colchicine is not a general pain relief drug, and is not used to treat pain in other disorders.[6]
Contraindications
Long-term (prophylactic) regimens of oral colchicine are absolutely contraindicated in patients with advanced renal failure (including those on dialysis).[6] About 10-20 percent of a colchicine dose is excreted unchanged by the kidneys; it is not removed by hemodialysis. Cumulative toxicity is a high probability in this clinical setting, and a severe neuromyopathy may result. The presentation includes a progressive onset of proximal weakness, elevated creatine kinase, and sensorimotor polyneuropathy. Colchicine toxicity can be potentiated by the concomitant use of cholesterol-lowering drugs.[6]
Adverse effects
Deaths – both accidental and intentional – have resulted from overdose of colchicine.[6] Typical side effects of moderate doses may include gastrointestinal upset, diarrhea, and neutropenia.[2] High doses can also damage bone marrow, lead to anemia, and cause hair loss. All of these side effects can result from inhibition of mitosis,[18] which may include neuromuscular toxicity and rhabdomyolysis.[6]
Toxicity
According to one review, colchicine poisoning by overdose (range of acute doses of 7 to 26 mg) occurs in three phases: 1) a gastrointestinal phase occurring 10-24 hours after ingestion; 2) a phase having poor prognosis involving multiple organ dysfunction occurring 24 hours to 7 days after ingestion, potentially evolving into rapid, progressive multi-organ failure and sepsis; 3) several weeks would be needed for a complete recovery if there are no complications.[19]
Colchicine can be toxic when ingested, inhaled, or absorbed in the eyes.[2] Colchicine can cause a temporary clouding of the cornea and be absorbed into the body, causing systemic toxicity. Symptoms of colchicine overdose start 2 to 24 hours after the toxic dose has been ingested and include burning in the mouth and throat, fever, vomiting, diarrhea, and abdominal pain.[6] This can cause hypovolemic shock due to extreme vascular damage and fluid loss through the gastrointestinal tract, which can be fatal.[19][20]
If the affected person does not recover, a multiple-system organ failure phase of colchicine overdose may result. This includes kidney damage, which causes low urine output and bloody urine; low white blood cell counts that can last for several days; anemia; muscular weakness; liver failure; hepatomegaly; bone marrow suppression; thrombocytopenia; and ascending paralysis leading to potentially fatal respiratory failure. Neurologic symptoms are also evident, including seizures, confusion, and delirium; children may experience hallucinations. Recovery may begin within six to eight days and begins with rebound leukocytosis and alopecia as organ functions return to normal.[19][18] Long-term exposure to colchicine can lead to toxicity, particularly of the bone marrow, kidney, and nerves. Effects of long-term colchicine toxicity include agranulocytosis, thrombocytopenia, low white blood cell counts, aplastic anemia, alopecia, rash, purpura, vesicular dermatitis, kidney damage, anuria, peripheral neuropathy, and myopathy.[18]
No specific antidote for colchicine is known, but supportive care is used in cases of overdose. In the immediate period after an overdose, monitoring for gastrointestinal symptoms, cardiac dysrhythmias, and respiratory depression is appropriate,[18] and may require gastrointestinal decontamination with activated charcoal or gastric lavage.[19][20]
Mechanism of toxicity
With overdoses, colchicine becomes toxic as an extension of its cellular mechanism of action via binding to tubulin.[19] Cells so affected undergo impaired protein assembly with reduced endocytosis, exocytosis, cellular motility, and interrupted function of heart cells, culminating in multi-organ failure.[3][19]
Drug interactions
Colchicine interacts with the P-glycoprotein transporter, and the CYP3A4 enzyme involved in drug and toxin metabolism.[6][19] Fatal drug interactions have occurred when colchicine was taken with other drugs that inhibit P-glycoprotein and CYP3A4, such as erythromycin or clarithromycin.[6]
People taking macrolide antibiotics, such as ketoconazole and cyclosporine for liver or kidney disease, should not take colchicine, as these drugs may interfere with colchicine metabolism and raise its blood levels, potentially increasing its toxicity abruptly.[6][19] Symptoms of toxicity include gastrointestinal upset, fever, muscle pain, low blood cell counts, and organ failure.[2][6] People with HIV/AIDS taking atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, or saquinavir may experience colchicine toxicity.[6] Grapefruit juice and statins can also increase colchicine concentrations.[6]
History
The plant source of colchicine, the autumn crocus (Colchicum autumnale), was described for treatment of rheumatism and swelling in the Ebers Papyrus (circa 1500 BC), an Egyptian medical papyrus.[21] Colchicum extract was first described as a treatment for gout in De Materia Medica by Pedanius Dioscorides, in the first century AD. Use of the bulb-like corms of Colchicum to treat gout probably dates to around 550 AD, as the "hermodactyl" recommended by Alexander of Tralles. Colchicum corms were used by the Persian physician Avicenna, and were recommended by Ambroise Pare in the 16th century, and appeared in the London Pharmacopoeia of 1618.[22] Colchicum plants were brought to North America by Benjamin Franklin, who suffered from gout himself and had written humorous doggerel about the disease during his stint as United States Ambassador to France.[23]
Colchicine was first isolated in 1820 by the French chemists P. S. Pelletier and J. B.Caventou.[24] In 1833, P. L. Geiger purified an active ingredient, which he named colchicine.[25] The determination of colchicine's structure required decades, although in 1945, Michael Dewar made an important contribution when he suggested that, among the molecule's three rings, two were seven-member rings.[26] Its pain-relieving and anti-inflammatory effects for gout were linked to its ability to bind with tubulin.
United States Unapproved Drugs Initiative
An unintended consequence of the 2006 U.S. Food and Drug Administration (FDA) safety program called the Unapproved Drugs Initiative — through which the FDA sought more rigorous testing of efficacy and safety of colchicine and other unapproved drugs[27] — was a price increase of 2000 percent [28] for "a gout remedy so old that the ancient Greeks knew about its effects."[28] Under Unapproved Drugs Initiative small companies like URL Pharma — Philadelphia drugmaker — were rewarded with licenses for testing of medicines like colchicine. In 2009, the FDA reviewed a New Drug Application for colchicine submitted by URL Pharma. URL Pharma did the testing, gained FDA formal approval and was granted rights over colchicine. With this monopoly pricing power, the price of colchicine increased.
In 2012 Asia’s biggest drugmaker — Takeda Pharmaceutical Co. — acquired URL Pharma for $800 million including the rights to colchicine (brand name Colcrys) earning $1.2 billion in revenue by raising the price even more.[28]
Oral colchicine had been used for many years as an unapproved drug with no FDA-approved prescribing information, dosage recommendations, or drug interaction warnings.[29] On July 30, 2009 the FDA approved colchicine as a monotherapy for the treatment of three different indications (familial Mediterranean fever, acute gout flares, and for the prophylaxis of gout flares[29]), and gave URL Pharma a three-year marketing exclusivity agreement[30] in exchange for URL Pharma doing 17 new studies and investing $100 million into the product, of which $45 million went to the FDA for the application fee. URL Pharma raised the price from $0.09 per tablet to $4.85, and the FDA removed the older unapproved colchicine from the market in October 2010, both in oral and intravenous forms, but gave pharmacies the opportunity to buy up the older unapproved colchicine.[31] Colchicine in combination with probenecid has been FDA-approved prior to 1982.[30]
In August 2009, colchicine won FDA approval in the United States as a stand-alone drug for the treatment of acute flares of gout and familial Mediterranean fever.[32][33] It had previously been approved as an ingredient in an FDA-approved combination product for gout. The approval was based on a study in which two doses (1.2 mg and 0.6 mg) an hour apart were as effective as higher doses in combating the acute flare of gout.[11]
Marketing exclusivity in the United States
As a drug antedating the FDA, colchicine was sold in the United States for many years without having been reviewed by the FDA for safety and efficacy. The FDA reviewed approved colchicine for gout flares, awarding Colcrys a three-year term of market exclusivity, prohibiting generic sales, and increasing the price of the drug from $0.09 to $4.85 per tablet.[34][35][36]
Numerous consensus guidelines, and previous randomized controlled trials, had concluded that colchicine is effective for acute flares of gouty arthritis. However, as of 2006, the drug was not formally approved by the FDA, owing to the lack of a conclusive randomized control trial (RCT). Through the Unapproved Drugs Initiative, the FDA sought more rigorous testing of efficacy and safety of colchicine and other unapproved drugs.[27] In exchange for paying for the costly testing, the FDA gave URL Pharma three years of market exclusivity for its Colcrys brand,[37] under the Hatch-Waxman Act, based in part on URL-funded research in 2007, including pharmacokinetic studies and a randomized control trial with 185 patients with acute gout.
In April 2010, an editorial in the New England Journal of Medicine said that the rewards of this legislation are not calibrated to the quality or value of the information produced, that no evidence of meaningful improvement to public health was seen, and that it would be less expensive for the FDA, the National Institutes of Health or large insurers to pay for trials themselves. Furthermore, the cost burden of this subsidy falls primarily on patients or their insurers.[38] In September 2010, the FDA ordered a halt to marketing unapproved single-ingredient oral colchicine.[39]
Colchicine patents expire on February 10, 2029.[40]
Orphan drug
URL Pharma also received seven years of market exclusivity for Colcrys in treatment of familial Mediterranean fever, under the Orphan Drug Law. URL Pharma then raised the price per tablet from $0.09 to $4.85 and sued to remove other versions from the market, increasing annual costs for the drug to U.S. state Medicaid programs from $1 million to $50 million. Medicare also paid significantly higher costs—making this a direct money-loser for the government. (In a similar case, thalidomide was approved in 1998 as an orphan drug for leprosy and in 2006 for multiple myeloma.)[38]
Biosynthesis
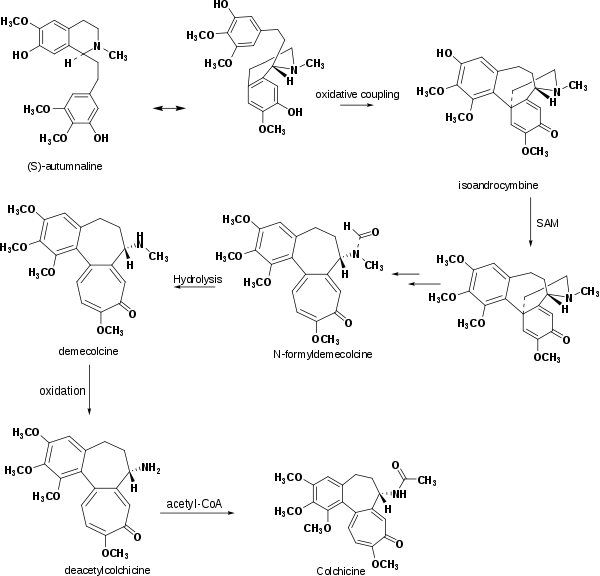
Several experiments show that the biosynthesis of colchicine involves the amino acids phenylalanine and tyrosine as precursors. Indeed, the feeding of C. autumnale with radioactive amino acid, tyrosine-2-C14, caused the latter to partially incorporate in the ring system of colchicine. The induced absorption of radioactive phenylalanine-2-C14 by C. byzantinum, another plant of the Colchicaceae family, resulted in its efficient absorption by colchicine.[41] However, it was proven that the tropolone ring of colchicine resulted, in essence, from the expansion of the tyrosine ring. Further radioactive feeding experiments of C. autumnale revealed that colchicine can be synthesized biosynthetically from (S)-autumnaline. That biosynthesic pathway occurs primarily through a para-para phenolic coupling reaction involving the intermediate isoandrocymbine. The resulting molecule undergoes O-methylation directed by S-adenosylmethionine. Two oxidation steps followed by the cleavage of the cyclopropane ring leads to the formation of the tropolone ring contained by N-formyldemecolcine. N-formyldemecolcine hydrolyzes then to generate the molecule demecolcine, which also goes through an oxidative demethylation that generates deacetylcolchicine. The molecule of colchicine appears finally after addition of acetyl-coenzyme A to deacetylcolchicine.[42][43]
Botanical use
Since chromosome segregation is driven by microtubules, colchicine is also used for inducing polyploidy in plant cells during cellular division by inhibiting chromosome segregation during meiosis; half the resulting gametes, therefore, contain no chromosomes, while the other half contains double the usual number of chromosomes (i.e., diploid instead of haploid, as gametes usually are), and lead to embryos with double the usual number of chromosomes (i.e., tetraploid instead of diploid). While this would be fatal in most higher animal cells, in plant cells it is not only usually well tolerated, but also frequently results in larger, hardier, faster-growing, and in general more desirable plants than the normally diploid parents; for this reason, this type of genetic manipulation is frequently used in breeding plants commercially.
When such a tetraploid plant is crossed with a diploid plant, the triploid offspring are usually sterile (unable to produce fertile seeds or spores), although many triploids can be propagated vegetatively. Growers of annual triploid plants not readily propagated must buy fresh seed from a supplier each year. Many sterile triploid plants, including some tree and shrubs, are becoming increasingly valued in horticulture and landscaping because they do not become invasive species. In certain species, colchicine-induced triploidy has been used to create "seedless" fruit, such as seedless watermelons (Citrullus lanatus). Since most triploids do not produce pollen themselves, such plants usually require cross-pollination with a diploid parent to induce fruit production.
Colchicine's ability to induce polyploidy can be also exploited to render infertile hybrids fertile, for example in breeding triticale (× Triticosecale) from wheat (Triticum spp.) and rye (Secale cereale). Wheat is typically tetraploid and rye diploid, with their triploid hybrid infertile; treatment of triploid triticale with colchicine gives fertile hexaploid triticale.
When used to induce polyploidy in plants, colchicine cream is usually applied to a growth point of the plant, such as an apical tip, shoot, or sucker. Also, seeds can be presoaked in a colchicine solution before planting. Another way to induce polyploidy is to chop off the tops of plants and carefully examine the regenerating lateral shoots and suckers to see if any look different.[44] If no visual difference is evident, flow cytometry can be used for analysis.
Doubling of plant chromosome numbers also occurs spontaneously in nature, with many familiar plants being fertile polyploids.[45] Natural hybridization between fertile parental plants of different levels of polyploidy can produce new plants at an intermediate level, such as a triploid produced by crossing between a diploid and a tetraploid, or a hexaploid produced by crossing between a tetraploid and an octoploid.
Regulation
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[46]
In popular culture
The toxicity of colchicine is illustrated in the episode Occam’s Razor of the TV series House.
References
- ↑ Shekelle PG, Newberry SJ, FitzGerald JD, Motala A, O'Hanlon CE, Tariq A, et al. (January 2017). "Management of Gout: A Systematic Review in Support of an American College of Physicians Clinical Practice Guideline". Annals of Internal Medicine. 166 (1): 37–51. doi:10.7326/M16-0461. PMID 27802478.
- 1 2 3 4 5 6 7 8 "Colcrys (colchicine, USP) tablets 0.6 mg. Drug Approval Package". US Food and Drug Administration. 17 February 2010. Retrieved 19 August 2018.
- 1 2 3 4 5 6 7 8 9 10 Dalbeth N, Lauterio TJ, Wolfe HR (October 2014). "Mechanism of action of colchicine in the treatment of gout". Clinical Therapeutics. 36 (10): 1465–79. doi:10.1016/j.clinthera.2014.07.017. PMID 25151572.
- 1 2 "Colchicine for acute gout: updated information about dosing and drug interactions". National Prescribing Service, Australia. 14 May 2010. Retrieved 14 May 2010.
- 1 2 "Colcrys (colchicine). Summary review for regulatory action" (PDF). Center for Drug Evaluation and Research, US Food and Drug Administration. 30 July 2009. Retrieved 19 August 2018.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 "Colchicine". Drugs.com. 1 January 2017. Retrieved 19 August 2018.
- ↑ Chen LX, Schumacher HR (October 2008). "Gout: an evidence-based review". Journal of Clinical Rheumatology. 14 (5 Suppl): S55–62. doi:10.1097/RHU.0b013e3181896921. PMID 18830092.
- ↑ "Information for Healthcare Professionals: New Safety Information for Colchicine (marketed as Colcrys)". U.S. Food and Drug Administration.
- ↑ Laubscher T, Dumont Z, Regier L, Jensen B (December 2009). "Taking the stress out of managing gout". Canadian Family Physician Medecin De Famille Canadien. 55 (12): 1209–12. PMC 2793228. PMID 20008601.
- 1 2 3 4 van Echteld I, Wechalekar MD, Schlesinger N, Buchbinder R, Aletaha D (August 2014). "Colchicine for acute gout". The Cochrane Database of Systematic Reviews. 8 (8): CD006190. doi:10.1002/14651858.CD006190.pub2. PMID 25123076.
- 1 2 Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW (April 2010). "High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study". Arthritis and Rheumatism. 62 (4): 1060–8. doi:10.1002/art.27327. PMID 20131255.
- 1 2 3 Cocco G, Chu DC, Pandolfi S (December 2010). "Colchicine in clinical medicine. A guide for internists". European Journal of Internal Medicine. 21 (6): 503–8. doi:10.1016/j.ejim.2010.09.010. PMID 21111934.
- ↑ Puéchal X, Terrier B, Mouthon L, Costedoat-Chalumeau N, Guillevin L, Le Jeunne C (March 2014). "Relapsing polychondritis". Joint, Bone, Spine. 81 (2): 118–24. doi:10.1016/j.jbspin.2014.01.001. PMID 24556284.
- ↑ Alabed S, Cabello JB, Irving GJ, Qintar M, Burls A (August 2014). "Colchicine for pericarditis". The Cochrane Database of Systematic Reviews. 8 (8): CD010652. doi:10.1002/14651858.CD010652.pub2. PMID 25164988.
- ↑ Portincasa P (2016). "Colchicine, Biologic Agents and More for the Treatment of Familial Mediterranean Fever. The Old, the New, and the Rare". Current Medicinal Chemistry. 23 (1): 60–86. PMID 26572612.
- ↑ Lennerz C, Barman M, Tantawy M, Sopher M, Whittaker P (December 2017). "Colchicine for primary prevention of atrial fibrillation after open-heart surgery: Systematic review and meta-analysis". International Journal of Cardiology. 249: 127–137. doi:10.1016/j.ijcard.2017.08.039. PMID 28918897.
- 1 2 "Colchicine images". Drugs.com. 6 August 2018. Retrieved 21 August 2018.
- 1 2 3 4 "CDC - The Emergency Response Safety and Health Database: Biotoxin: Cochicine". Centers for Disease Control and Prevention, US Department of Health and Human Services. Retrieved 31 December 2015.
- 1 2 3 4 5 6 7 8 Finkelstein Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, Pollak U, Koren G, Bentur Y (June 2010). "Colchicine poisoning: the dark side of an ancient drug". Clinical Toxicology. 48 (5): 407–14. doi:10.3109/15563650.2010.495348. PMID 20586571.
- 1 2 Matt Doogue (2014). "Colchicine – extremely toxic in overdose" (PDF). Christchurch and Canterbury District Health Board, New Zealand. Retrieved 23 August 2018.
- ↑ Graham W, Roberts JB (March 1953). "Intravenous colchicine in the management of gouty arthritis" (PDF). Annals of the Rheumatic Diseases. 12 (1): 16–9. doi:10.1136/ard.12.1.16. PMC 1030428. PMID 13031443.
- ↑ Hartung EF (September 1954). "History of the use of colchicum and related medicaments in gout; with suggestions for further research" (PDF). Annals of the Rheumatic Diseases. 13 (3): 190–200. doi:10.1136/ard.13.3.190. PMC 1006735. PMID 13198053. (free BMJ registration required)
- ↑ Ebadi MS (2007). Pharmacodynamic basis of herbal medicine. ISBN 978-0-8493-7050-2.
- ↑ Pelletier and Caventou (1820) "Examen chimique des plusieurs végétaux de la famille des colchicées, et du principe actif qu'ils renferment. [Cévadille (veratrum sabadilla) ; hellébore blanc (veratrum album) ; colchique commun (colchicum autumnale)]" (Chemical examination of several plants of the meadow saffron family, and of the active principle that they contain.) Annales de Chimie et de Physique, 14 : 69-81.
- ↑ Geiger, Ph. L. (1833) "Ueber einige neue giftige organische Alkalien" (On some new poisonous organic alkalis) Annalen der Pharmacie, 7 (3) : 269-280; colchicine is discussed on pages 274-276.
- ↑ Dewar MJ (February 3, 1945). "Structure of colchicine". Letters to Editor. Nature. 155: 141–142. Dewar did not prove the structure of colchicine; he merely suggested that it contained two seven-membered rings. Colchicine's structure was determined by X-ray crystallography in 1952 King MV, de Vries JL, Pepinsky R (July 1952). "An x-ray diffraction determination of the chemical structure of colchicine". Acta Crystallographica. 5: 437–440. Its total synthesis was first accomplished in 1959 Eschenmoser A (1959). "Synthese des Colchicins". Angewandte Chemie. 71: 637–640.
- 1 2 "FDA Unapproved Drugs Initiative".
- 1 2 3 Langreth R, Koons C (6 October 2015). "2,000% Drug Price Surge Is a Side Effect of FDA Safety Program". Bloomberg. Retrieved 27 October 2015.
- 1 2 "FDA Approves Colchicine With Drug Interaction and Dose Warnings". July 2009.
- 1 2 "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". fda.gov.
- ↑ "Questions and Answers for Patients and Healthcare Providers Regarding Single-ingredient Oral Colchicine Products". fda.gov.
- ↑ "FDA Approves Gout Treatment After Long Years of Use". medpagetoday.com. 3 August 2009.
- ↑ Cerquaglia C, Diaco M, Nucera G, La Regina M, Montalto M, Manna R (February 2005). "Pharmacological and clinical basis of treatment of Familial Mediterranean Fever (FMF) with colchicine or analogues: an update". Current Drug Targets. Inflammation and Allergy. 4 (1): 117–24. doi:10.2174/1568010053622984. PMID 15720245.
- ↑ Karst KR (21 October 2009). "California Court Denies Preliminary Injunction in Lanham Act Case Concerning Unapproved Colchicine Drugs".
- ↑ Meyer H (29 December 2009). "The High Price of FDA Approval". Kaiser Health News and the Philadelphia Inquirer.
- ↑ Colcrys vs. Unapproved Colchicine Statement from URL Pharma
- ↑ "About Colcrys". Colcrys. URL Pharma. Retrieved 11 September 2011.
- 1 2 Kesselheim AS, Solomon DH (June 2010). "Incentives for drug development--the curious case of colchicine". The New England Journal of Medicine. 362 (22): 2045–7. doi:10.1056/NEJMp1003126. PMID 20393164.
- ↑ "FDA orders halt to marketing of unapproved single-ingredient oral colchicine". 30 September 2010.
- ↑ "Generic Colcrys Availability". drugs.com.
- ↑ Leete E (1963). "The biosynthesis of the alkaloids of Colchicum: The incorporation of phenylalaline-2-C14 into colchicine and demecolcine". J. Am. Chem. Soc. 85 (22): 3666–3669. doi:10.1021/ja00905a030.
- ↑ Dewick PM (2009). Medicinal Natural Products: A biosynthetic Approach. Wiley. pp. 360–362.
- ↑ Maier UH, Meinhart HZ (1997). "Colchicine is formed by para para phenol-coupling from autumnaline". Tetrahedron Lett. 38 (42): 7357–7360. doi:10.1016/s0040-4039(97)10011-9.
- ↑ Deppe C (1993). Breed Your own Vegetable Varieties. Little, Brown & Company. pp. 150–151. ISBN 0-316-18104-8.
- ↑ Derman H, Emsweller SL. "The use of colchicine in plant breeding". archive.org. Retrieved 26 April 2016.
- ↑ "40 CFR Appendix A to Part 355, The List of Extremely Hazardous Substances and Their Threshold Planning Quantities". LII / Legal Information Institute. Retrieved 2018-03-11.
External links
| Wikimedia Commons has media related to Colchicine. |
- Dowd, Matthew J. (April 30, 1998). "Colchicine". Virginia Commonwealth University. Archived from the original on 2010-06-10.
- NIOSH Emergency Response Database