Leptospirosis
| Leptospirosis | |
|---|---|
| Synonyms | Field fever,[1] rat catcher's yellows,[2] pretibial fever[3] |
 | |
| Leptospira magnified 200-fold with dark-field microscope | |
| Specialty | Infectious disease |
| Symptoms | None, headaches, muscle pains, fevers[4] |
| Complications | Bleeding from the lungs, meningitis, kidney failure[4][5] |
| Causes | Leptospira typically spread by rodents[4][6] |
| Diagnostic method | Testing blood for antibodies against the bacterium or its DNA[7] |
| Differential diagnosis | Malaria, enteric fever, rickettsiosis, dengue[8] |
| Treatment | Doxycycline, penicillin, ceftriaxone[4] |
| Frequency | ~8.5 million people per year[9] |
| Deaths | Unknown[9] |
Leptospirosis is an infection caused by corkscrew-shaped bacteria called Leptospira.[4] Signs and symptoms can range from none to mild such as headaches, muscle pains, and fevers; to severe with bleeding from the lungs or meningitis.[4][5] If the infection causes the person to turn yellow, have kidney failure and bleeding, it is then known as Weil's disease.[5] If it also causes bleeding into the lungs then it is known as severe pulmonary hemorrhage syndrome.[5]
Up to 13 different genetic types of Leptospira may cause disease in humans.[10] It is transmitted by both wild and domestic animals.[5] The most common animals that spread the disease are rodents.[6] It is often transmitted by animal urine or by water or soil containing animal urine coming into contact with breaks in the skin, eyes, mouth, or nose.[4][11] In the developing world the disease most commonly occurs in farmers and low-income people who live in cities.[5] In the developed world it most commonly occurs in those involved in outdoor activities in warm and wet areas of the world.[4] Diagnosis is typically by looking for antibodies against the bacterium or finding its DNA in the blood.[7]
Efforts to prevent the disease include protective equipment to prevent contact when working with potentially infected animals, washing after this contact, and reducing rodents in areas people live and work.[4] The antibiotic doxycycline, when used in an effort to prevent infection among travellers, is of unclear benefit.[4] Vaccines for animals exist for certain type of Leptospira which may decrease the risk of spread to humans.[4] Treatment if infected is with antibiotics such as: doxycycline, penicillin, or ceftriaxone.[4] Weil's disease and severe pulmonary haemorrhage syndrome result in death rates greater than 10% and 50%, respectively, even with treatment.[5]
It is estimated that seven to ten million people are infected by leptospirosis per year.[9] The number of deaths this causes is not clear.[9] The disease is most common in tropical areas of the world but may occur anywhere.[4] Outbreaks may occur in slums of the developing world.[5] The disease was first described by physician Adolf Weil in 1886 in Germany.[4][12] Animals which are infected may have no symptoms, mild symptoms, or severe symptoms.[10] Symptoms may vary by the type of animal.[10] In some animals Leptospira live in the reproductive tract, leading to transmission during mating.[13]
Signs and symptoms
Leptospiral infection in humans causes a range of symptoms, and some infected persons may have no symptoms at all. Leptospirosis is a biphasic disease that begins suddenly with fever accompanied by chills, intense headache, severe myalgia (muscle ache), abdominal pain, conjunctival suffusion (red eye), and occasionally a skin rash.[14] The symptoms appear after an incubation period of 7–12 days. The first phase (acute or septic phase) ends after 3–7 days of illness.[15] The disappearance of symptoms coincides with the appearance of antibodies against Leptospira and the disappearance of all the bacteria from the bloodstream. The patient is asymptomatic for 3–4 days until the second phase begins with another episode of fever.[14] The hallmark of the second phase is meningitis (inflammation of the membranes covering the brain).[16]
Ninety percent of cases of the disease are mild leptospirosis. The rest experience severe disease, which develops during the second stage or occurs as a single progressive illness.[17] The classic form of severe leptospirosis is known as Weil's disease, which is characterized by liver damage (causing jaundice), kidney failure, and bleeding.[18] Additionally, the heart and brain can be affected, meningitis of the outer layer of the brain, encephalitis of brain tissue with same signs and symptoms; and lung affected as the most serious and life-threatening of all leptospirosis complications. The infection is often incorrectly diagnosed due to the nonspecific symptoms.
Other severe manifestations include extreme fatigue, hearing loss, respiratory distress, and azotemia.
Cause
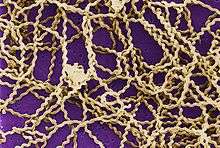
Leptospirosis is caused by spirochaete bacteria belonging to the genus Leptospira. 21 species of Leptospira have been identified.[10] 13 species cause disease or have been detected in human cases.[10][19]
Leptospira are also classified based on their serovar. About 250 pathogenic serovars of Leptospira are recognized. The diverse sugar composition of the lipopolysaccharide on the surface of the spirochete is responsible for the antigenic difference between serovars. Antigenically related serovars are grouped into 24 serogroups, which are identified using the microscopic agglutination test (MAT). A given serogroup is often found in more than one species, suggesting that the LPS genes that determine the serovar are exchanged between species.[19]
The traditional serologic system currently seems more useful from a diagnostic and epidemiologic standpoint—but this may change with further development and spread of technologies like polymerase chain reaction (PCR).
Transmission
Leptospirosis is transmitted by the urine of an infected animal and is contagious as long as the urine is still moist. Although Leptospira has been detected in reptiles and birds, only mammals are able to transmit the bacterium to humans and other animals.[20] Rats, mice, and moles are important primary hosts—but a wide range of other mammals including dogs, deer, rabbits, hedgehogs, cows, sheep, swine, raccoons, opossums, skunks, and certain marine mammals carry and transmit the disease as secondary hosts. In Africa, the banded mongoose has been identified as a carrier of the pathogen, likely in addition to other African wildlife hosts.[21] Dogs may lick the urine of an infected animal off the grass or soil, or drink from an infected puddle.
House-bound domestic dogs have contracted leptospirosis, apparently from licking the urine of infected mice in the house. The type of habitats most likely to carry infective bacteria includes muddy riverbanks, ditches, gullies, and muddy livestock rearing areas where there is a regular passage of wild or farm mammals. The incidence of leptospirosis correlates directly with the amount of rainfall, making it seasonal in temperate climates and year-round in tropical climates. Leptospirosis also transmits via the semen of infected animals.[22]
Humans become infected through contact with water, food, or soil that contains urine from these infected animals. This may happen by swallowing contaminated food or water or through skin contact. The disease is not known to spread between humans, and bacterial dissemination in convalescence is extremely rare in humans. Leptospirosis is common among water-sport enthusiasts in specific areas, as prolonged immersion in water promotes the entry of this bacterium. Surfers and whitewater paddlers[23] are at especially high risk in areas that have been shown to contain these bacteria, and can contract the disease by swallowing contaminated water, splashing contaminated water into their eyes or nose, or exposing open wounds to infected water.[24] In tropical and semi tropical areas, like South and South-east Asia the disease is increasingly seen as epidemics after heavy rains, sometimes after flooding. Periods of heavy rain followed by days of little or no rain seemed to be the setting for leptospirosis epidemics in this part of the world. Most cases seemed to occur by cutaneous exposure of the legs while walking in stagnant water or moist soil. [25] The presence of wounds or fissures on the legs are a risk factor for acquiring the infection.
At-risk occupations
Occupations at risk include veterinarians, slaughterhouse workers, countryside rangers, farmers, sailors on rivers, sewer maintenance workers, waste disposal facility workers, and people who work on derelict buildings.[26] Slaughterhouse workers can contract the disease through contact with infected blood or body fluids. Rowers, kayakers and canoeists also sometimes contract the disease.[27] It was once mostly work-related but is now often also related to adventure tourism and recreational activities.[5]
Diagnosis

On infection the microorganism can be found in blood and cerebrospinal fluid (CSF) for the first 7 to 10 days (invoking serologically identifiable reactions) and then moving to the kidneys. After 7 to 10 days the microorganism can be found in fresh urine. Hence, early diagnostic efforts include testing a serum or blood sample serologically with a panel of different strains.
Kidney function tests (blood urea nitrogen and creatinine) as well as blood tests for liver functions are performed. The latter reveal a moderate elevation of transaminases. Brief elevations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyltransferase (GGT) levels are relatively mild. These levels may be normal, even in children with jaundice.
Diagnosis of leptospirosis is confirmed with tests such as enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR). The MAT (microscopic agglutination test), a serological test, is considered the gold standard in diagnosing leptospirosis. As a large panel of different leptospira must be subcultured frequently, which is both laborious and expensive, it is underused, especially in developing countries.
Differential diagnosis list for leptospirosis is very large due to diverse symptoms. For forms with middle to high severity, the list includes dengue fever and other hemorrhagic fevers, hepatitis of various causes, viral meningitis, malaria, and typhoid fever. Light forms should be distinguished from influenza and other related viral diseases. Specific tests are a must for proper diagnosis of leptospirosis.
Under circumstances of limited access (e.g., developing countries) to specific diagnostic means, close attention must be paid to the medical history of the patient. Factors such as certain dwelling areas, seasonality, contact with stagnant contaminated water (bathing, swimming, working on flooded meadows, etc.) or rodents in the medical history support the leptospirosis hypothesis and serve as indications for specific tests (if available).
Leptospira can be cultured in Ellinghausen-McCullough-Johnson-Harris medium (EMJH), which is incubated at 28 to 30 °C.[28] The median time to positivity is three weeks with a maximum of three months. This makes culture techniques useless for diagnostic purposes but is commonly used in research.
Prevention
Doxycycline has been provided once a week as a prophylaxis to minimize infections during outbreaks in endemic regions.[29] However, there is no evidence that chemoprophylaxis is effective in containing outbreaks of leptospirosis,[30] and use of antibiotics increases antibiotics resistance. Pre-exposure prophylaxis may be beneficial for individuals traveling to high-risk areas for a short stay.[31]
Effective rat control and avoidance of urine contaminated water sources are essential preventive measures. Human vaccines are available only in a few countries, such as Cuba and China.[5] Animal vaccines only cover a few strains of the bacteria. Dog vaccines are effective for at least one year.[32]
Treatment
Effective antibiotics include penicillin G, ampicillin, amoxicillin and doxycycline. In more severe cases cefotaxime or ceftriaxone should be preferred.
Glucose and salt solution infusions may be administered; dialysis is used in serious cases. Elevations of serum potassium are common and if the potassium level gets too high special measures must be taken. Serum phosphorus levels may likewise increase to unacceptable levels due to kidney failure.
Treatment for hyperphosphatemia consists of treating the underlying disease, dialysis where appropriate, or oral administration of calcium carbonate, but not without first checking the serum calcium levels (these two levels are related). Administration of corticosteroids in gradually reduced doses (e.g., prednisolone) for 7–10 days is recommended by some specialists in cases of severe hemorrhagic effects. Organ-specific care and treatment are essential in cases of kidney, liver, or heart involvement.
Epidemiology
It is estimated that seven to ten million people are infected by leptospirosis annually.[9] One million cases of severe leptospirosis occur annually, with 58,900 deaths.[33] Annual rates of infection vary from 0.02 per 100,000 in temperate climates to 10 to 100 per 100,000 in tropical climates.[29][34] This leads to a lower number of registered cases than likely exists.
The number of new cases of leptospirosis is difficult to estimate since many cases of the disease go unreported. There are many reasons for this, but the biggest issue is separating the disease from other similar conditions.[34] Laboratory testing is lacking in many areas.[34]
In context of global epidemiology, the socioeconomic status of many of the world’s population is closely tied to malnutrition; subsequent lack of micronutrients may lead to increased risk of infection and death due to leptospirosis infection.[35] Micronutrients such as iron, calcium, and magnesium represent important areas of future research.[35]
Outbreaks that occurred after the 1940s have happened mostly in the late summer seasons, which happens to be the driest part of the year. The people at the highest risk for leptospirosis are young people whose age ranges from 5–16 years old, and can also range to young adults.[36]
The amount of cases increase during the rainy season in the tropics and during the late summer or early fall in Western countries. This happens because leptospires survive best in fresh water, damp alkaline soil, vegetation, and mud with temperatures higher than 22 °C.[37] This also leads to increased risk of exposure to populations during flood conditions, and leptospire concentrations to peak in isolated pools during drought. There is no evidence of leptospirosis having any effect on sexual and age-related differences.[37] However, a major risk factor for development of the disease is occupational exposure, a disproportionate number of working-aged males are affected.[37] There have been reported outbreaks where more than 40% of people are younger than 15. “Active surveillance measures have detected leptospire antibodies in as many as 30% of children in some urban American populations.”[37] Potential reasons for such cases include children playing with suspected vectors such as dogs or indiscriminate contact with water.
History
The disease was first described by Adolf Weil in 1886 when he reported an "acute infectious disease with enlargement of spleen, jaundice, and nephritis."[12] Leptospira was first observed in 1907 from a post mortem renal tissue slice.[38] In 1908, Inada and Ito first identified it as the causative organism[39] and in 1916 noted its presence in rats.[40]
Leptospirosis was postulated as the cause of an epidemic among American Indians along the coast of Massachusetts which occurred immediately before the arrival of the Pilgrims in 1620 and killed most of the population.[41] Earlier proposals included plague, yellow fever, smallpox, influenza, chickenpox, typhus, typhoid fever, trichinellosis, meningitis, and syndemic infection of hepatitis B with hepatitis D.[42][43][44][45] The disease may have been brought to the New World by Europeans and spread by the daily activities of the Indians.[41]
Before Weil's characterization in 1886, the disease known as infectious jaundice was very likely the same as Weil's disease or severe icteric leptospirosis. During the Egyptian campaign, Napoleon's army suffered from what was probably infectious jaundice.[46] Infectious jaundice occurred among troops during the American Civil War.[47]
It was also reported among troops at Gallipoli and other battles of World War I, where the sodden conditions of trench warfare favored infection. Terms used in early 20th century descriptions of leptospirosis include the pseudo-dengue of Java, seven-day fever, autumn fever, Akiyama disease, and marsh or swamp fever. L icterohaemorrhagiae was identified as the causative agent in pre-World War II outbreaks in Japan, which were characterized by jaundice and a high mortality rate.
In October 2010 British rower Andy Holmes died after contracting Weil's Disease.[48] His death has raised awareness of the disease among the public and medical professionals.[49]
Names
Leptospirosis has many different names including: "7-day fever",[1] "harvest fever",[1] "field fever",[1] "canefield fever",[1] "mild fever",[1] "rat catcher's yellows",[2] "Fort Bragg fever",[3] and "pretibial fever".[3]"rat fever"
It has historically been known as "black jaundice"[50] and in Japan it is called "nanukayami fever".[51] Weil's disease or Weil's syndrome is also known as spirochaetosis icterohaemorrhagica.[52]
Other animals
Incubation (time of exposure to first symptoms) in animals is anywhere from 2 to 20 days. In dogs, leptospirosis most often damages the liver and kidney. In addition, recent reports describe a pulmonary form of canine leptospirosis associated with severe hemorrhage in the lungs—similar to human pulmonary hemorrhagic syndrome.[53][54] Vasculitis may occur, causing edema and potentially disseminated intravascular coagulation (DIC). Myocarditis, pericarditis, meningitis, and uveitis are also possible sequelae.[27]
At least five important serovars exist in the United States and Canada, all of which cause disease in dogs:[27][53][54]
- Icterohaemorrhagiae
- Canicola
- Pomona
- Grippotyphosa
- Bratislava
In dogs when leptospirosis is caused by L. interrogans it may be referred to as "canicola fever".[51] Leptospirosis should be strongly suspected and included as part of a differential diagnosis if the sclerae of a dog's eyes appear jaundiced (even slightly yellow). The absence of jaundice does not eliminate the possibility of leptospirosis, and its presence could indicate hepatitis or other liver pathology rather than leptospirosis. Vomiting, fever, failure to eat, reduced urine output, unusually dark or brown urine, and lethargy are also indications of the disease.
In dogs, penicillin is most commonly used to end the leptospiremic phase (infection of the blood), and doxycycline is used to eliminate the carrier state.
References
- 1 2 3 4 5 6 Mosby's Medical Dictionary (9 ed.). Elsevier Health Sciences. 2013. p. 697. ISBN 9780323112581. Archived from the original on 8 September 2017.
- 1 2 McKay, James E. (2001). Comprehensive health care for dogs. Minnetonka, MN.: Creative Pub. International. p. 97. ISBN 9781559717830.
- 1 2 3 James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0. :290
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Slack, A (Jul 2010). "Leptospirosis". Australian Family Pphysician. 39 (7): 495–8. PMID 20628664.
- 1 2 3 4 5 6 7 8 9 10 McBride, AJ; Athanazio, DA; Reis, MG; Ko, AI (Oct 2005). "Leptospirosis". Current Opinion in Infectious Diseases. 18 (5): 376–86. doi:10.1097/01.qco.0000178824.05715.2c. PMID 16148523.
- 1 2 Wasiński B, Dutkiewicz J (2013). "Leptospirosis—current risk factors connected with human activity and the environment". Annals of Agricultural and Environmental Medicine. 20 (2): 239–44. PMID 23772568. Archived from the original on 14 September 2014.
- 1 2 Picardeau M (January 2013). "Diagnosis and epidemiology of leptospirosis". Médecine et Maladies Infectieuses. 43 (1): 1–9. doi:10.1016/j.medmal.2012.11.005. PMID 23337900.
- ↑ Farrar, Jeremy; Hotez, Peter; Junghanss, Thomas; Kang, Gagandeep; Lalloo, David; White, Nicholas J. (2013). Manson's Tropical Diseases E-Book. Elsevier Health Sciences. p. 438. ISBN 9780702053061. Archived from the original on 8 September 2017.
- 1 2 3 4 5 "Leptospirosis". NHS. 11 July 2012. Archived from the original on 14 March 2014. Retrieved 14 March 2014.
- 1 2 3 4 5 "Leptospirosis" (PDF). The Center for Food Security and Public Health. October 2013. Archived (PDF) from the original on 24 November 2014. Retrieved 8 November 2014.
- ↑ "Leptospirosis (Infection)". Centers for Disease Control and Prevention. Archived from the original on 11 October 2014. Retrieved 8 November 2014.
- 1 2 Weil, A. (1886). "Über eine eigenthümliche, mit Milztumor, Icterus und Nephritis einhergehende, acute Infektionskrankheit" [On a strange, acute infectious disease, accompanied by swelling of the spleen, icterus, and nephritis]. Deutsches Archiv für klinische Medizin (in German). 39: 209–232.
- ↑ Faine, Solly; Adler, Ben; Bolin, Carole (1999). "Clinical Leptospirosis in Animals". Leptospira and Leptospirosis (Revised 2nd ed.). Melbourne, Australia: MediSci. p. 113. ISBN 0-9586326-0-X.
- 1 2 Levett PN (April 2001). "Leptospirosis". Clinical Microbiology Reviews. 14 (2): 296–326. doi:10.1128/CMR.14.2.296-326.2001. PMC 88975. PMID 11292640.
- ↑ Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, Durski K, Hartskeerl RA (January 2014). "Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies". Diagnostic Microbiology and Infectious Disease. 78 (1): 1–8. doi:10.1016/j.diagmicrobio.2013.09.012. PMID 24207075.
- ↑ Farr RW (July 1995). "Leptospirosis". Clinical Infectious Diseases. 21 (1): 1–6, quiz 7–8. doi:10.1093/clinids/21.1.1. PMID 7578715.
- ↑ Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM (December 2003). "Leptospirosis: a zoonotic disease of global importance". The Lancet Infectious Diseases. 3 (12): 757–71. doi:10.1016/s1473-3099(03)00830-2. PMID 14652202.
- ↑ Ko AI, Goarant C, Picardeau M (October 2009). "Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen". Nature Reviews Microbiology. 7 (10): 736–47. doi:10.1038/nrmicro2208. PMC 3384523. PMID 19756012.
- 1 2 Cerqueira GM, Picardeau M (September 2009). "A century of Leptospira strain typing". Infection, Genetics and Evolution. 9 (5): 760–8. doi:10.1016/j.meegid.2009.06.009. PMID 19540362.
- ↑ Guerra MA (February 2009). "Leptospirosis". Journal of the American Veterinary Medical Association. 234 (4): 472–8, 430. doi:10.2460/javma.234.4.472. PMID 19222355.
- ↑ Jobbins SE, Sanderson CE, Alexander KA (March 2014). "Leptospira interrogans at the human-wildlife interface in northern Botswana: a newly identified public health threat". Zoonoses and Public Health. 61 (2): 113–23. doi:10.1111/zph.12052. PMID 23672285. Lay summary – Sciencedaily (2013-05-14).
- ↑ Kiktenko VS; Balashov, NG; Rodina, VN (1976). "Leptospirosis infection through insemination of animals". Journal of Hygiene, Epidemiology, Microbiology, and Immunology. 21 (2): 207–213. PMID 987112.
- ↑ Shaw RD (June 1992). "Kayaking as a risk factor for leptospirosis". Missouri Medicine. 89 (6): 354–7. PMID 1620089.
- ↑ "transworld.net: Seven Surfing Sicknesses". grindtv.com. Now on Nationwide tour. Archived from the original on 31 January 2009. .
- ↑ Pappachan MJ, Sheela M, Aravindan KP. Relation of rainfall pattern and epidemic leptospirosis in the Indian state of Kerala. J Epidemiol Community Health. 2004;58:1054.
- ↑ "Weil's Disease at Work". injury-compensation-zone.co.uk. Injury Compensation. Archived from the original on 3 December 2013.
- 1 2 3 Langston CE, Heuter KJ (July 2003). "Leptospirosis. A re-emerging zoonotic disease". Veterinary Clinics of North America: Small Animal Practice. 33 (4): 791–807. doi:10.1016/S0195-5616(03)00026-3. PMID 12910744.
- ↑ Rule PL, Alexander AD (1986). "Gellan gum as a substitute for agar in leptospiral media". Journal of Clinical Microbiology. 23 (3): 500–504. PMC 268682. PMID 3754265.
- 1 2 Pavli A, Maltezou HC (2008). "Travel-acquired leptospirosis". Journal of Travel Medicine. 15 (6): 447–53. doi:10.1111/j.1708-8305.2008.00257.x. PMID 19090801.
- ↑ "Leptospirosis: an emerging public health problem". Weekly Epidemiological Record. 86 (6): 45–50. February 2011. PMID 21302385.
- ↑ Brett-Major DM, Lipnick RJ (2009). "Antibiotic prophylaxis for leptospirosis". Cochrane Database of Systematic Reviews (3): CD007342. doi:10.1002/14651858.CD007342.pub2. PMID 19588424.
- ↑ Goldstein RE (November 2010). "Canine leptospirosis". Veterinary Clinics of North America: Small Animal Practice. 40 (6): 1091–101. doi:10.1016/j.cvsm.2010.07.008. PMID 20933138.
- ↑ Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI (2015). "Global Morbidity and Mortality of Leptospirosis: A Systematic Review". PLOS Neglected Tropical Diseases. 9 (9): e0003898. doi:10.1371/journal.pntd.0003898. PMC 4574773. PMID 26379143.
- 1 2 3 "WHO | Leptospirosis Burden Epidemiology Reference Group (LERG)". www.who.int. Retrieved 2017-11-30.
- 1 2 Herman, Heather S.; Mehta, Saurabh; Cárdenas, Washington B.; Stewart-Ibarra, Anna M.; Finkelstein, Julia L. (2016-07-07). "Micronutrients and Leptospirosis: A Review of the Current Evidence". PLOS Neglected Tropical Diseases. 10 (7): e0004652. doi:10.1371/journal.pntd.0004652. ISSN 1935-2735.
- ↑ Galton, Mildred M. (February 1959). "The epidemiology of leptospirosis in the United States". Public Health Reports. 74 (2): 141–148. ISSN 0094-6214. PMC 1929197. PMID 13623988.
- 1 2 3 4 "Leptospirosis: Practice Essentials, Background, Pathophysiology". 2017-11-17.
- ↑ Stimson, A.M. (1907). "Note on an organism found in yellow-fever tissue". Public Health Reports. 22 (18): 541. doi:10.2307/4559008.
- ↑ Inada R, Ito Y (1908). "A report of the discovery of the causal organism (a new species of spirocheta) of Weil's disease". Tokyo Ijishinshi. 1915: 351–60.
- ↑ Inanda R, Ido Y, Hoke R, Kaneko R, Ito H (1916). "The etiology, mode of infection and specific therapy of Weil's disease" (PDF). The Journal of Experimental Medicine. 23 (3): 377–402. doi:10.1084/jem.23.3.377. PMC 2125418.
- 1 2 Marr JS, Cathey JT (February 2010). "New hypothesis for cause of an epidemic among Native Americans, New England, 1616–1619". Emerging Infectious Diseases. 16 (2): 281–6. doi:10.3201/eid1602.090276. PMC 2957993. PMID 20113559. Archived from the original on 30 January 2010.
- ↑ Webster N (1799). A brief history of epidemic and pestilential diseases. Hartford CT: Hudson and Goodwin. Archived from the original on 5 January 2014.
- ↑ Williams H (1909). "The epidemic of the Indians of New England, 1616–1620, with remarks on Native American infections". The Johns Hopkins Hospital Bulletin. 20: 340–349.
- ↑ Bratton TL (1988). "The identity of the New England Indian epidemic of 1616–19". Bulletin of the History of Medicine. 62 (3): 351–383. PMID 3067787.
- ↑ Speiss A, Speiss BD (1987). "New England pandemic of 1616–1622. cause and archeological implication". Man in the Northeast. 34: 71–83.
- ↑ Edward Rhodes Stitt; Richard Pearson Strong (1944). Stitt's Diagnosis, prevention and treatment of tropical diseases (7th ed.). York, PA: Blakiston. Archived from the original on 29 June 2014.
- ↑ Neill M (1918). "The problem of acute infectious jaundice in the United States". Public Health Reports. 33 (19): 717–726.
- ↑ Leggat, David (27 October 2010). "Rowing: Rare disease kills rowing great". The New Zealand Herald. Retrieved 14 October 2011.
- ↑ Forbes AE, Zochowski WJ, Dubrey SW, Sivaprakasam V (July 2012). "Leptospirosis and Weil's disease in the UK". QJM : Monthly Journal of the Association of Physicians. 105 (12): 1151–62. doi:10.1093/qjmed/hcs145. PMID 22843698.
- ↑ David Clapham (2004). Small Water Supplies: A Practical Guide. Routledge. p. 125. ISBN 9781134457496. Archived from the original on 8 September 2017.
- 1 2 Dorland's illustrated medical dictionary (32nd ed.). Philadelphia: Elsevier/Saunders. 2012. p. 1231. ISBN 9781455709854. Archived from the original on 8 September 2017.
- ↑ Kassirsky, I.; Katz, N. Plotnikov ; translated from the Russian by Miriam; Creighton, H.C. (2003). Diseases of warm lands : a clinical manual. Honolulu: University Press of the Pacific. p. 193. ISBN 9781410207890. Archived from the original on 8 September 2017.
- 1 2 Klopfleisch R, Kohn B, Plog S, Weingart C, Nöckler K, Mayer-Scholl A, Gruber AD (2011). "An Emerging Pulmonary Haemorrhagic Syndrome in Dogs: Similar to the Human Leptospiral Pulmonary Haemorrhagic Syndrome?". Veterinary Medicine International. 33: 928541. doi:10.4061/2010/928541. PMC 3025382. PMID 21274452.
- 1 2 Kohn B, Steinicke K, Arndt G, Gruber AD, Guerra B, Jansen A, Kaser-Hotz B, Klopfleisch R, Lotz F, Luge E, Nöckler K (2010). "Pulmonary abnormalities in dogs with leptospirosis". Journal of Veterinary Internal Medicine. 24 (6): 1277–1282. doi:10.1111/j.1939-1676.2010.0585.x. PMID 20738768.
External links
| Classification | |
|---|---|
| External resources |
- "Leptospirosis". U.S. Disease Control and Prevention Center.
- "Leptospira". NCBI Taxonomy Browser. 171.