Loracarbef
 | |
| Clinical data | |
|---|---|
| Trade names | Lorabid |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601206 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 25% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
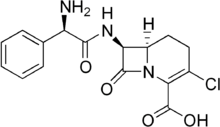
| Formula | C16H16ClN3O4 |
| Molar mass | 349.769 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Loracarbef is an antibiotic.[1] It is a carbacephem, but it is sometimes grouped together with the second-generation cephalosporin antibiotics. Loracarbef is a synthetic "carba" analog of cefaclor, and is more stable chemically.
History
Loracarbef received FDA approval in 1991 and it was marketed under the trade name Lorabid. Its use was discontinued in 2006.
Side effects
Diarrhea is the most common adverse effect with loracarbef. Side effects are more frequently seen with children under the age of twelve.
References
- ↑ Biedenbach DJ, Jones RN (February 1994). "Predictive accuracy of disk diffusion test for Proteus vulgaris and Providencia species against five newer orally administered cephalosporins, cefdinir, cefetamet, cefprozil, cefuroxime, and loracarbef". J. Clin. Microbiol. 32 (2): 559–62. PMC 263078. PMID 8150976.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.