Hydroxyprogesterone caproate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Delalutin, Proluton, Proluton Depot, Makena others |
| Synonyms | OHPC; Hydroxyprogesterone capronate; Hydroxyprogesterone hexanoate; 17α-Hydroxyprogesterone caproate; 17α-OHPC; 17-Hydroxyprogesterone caproate; 17-OHPC; 17-HPC; 17α-HPC; HPC; 17α-Hydroxypregn-4-ene-3,20-dione 17α-hexanoate |
| Pregnancy category |
|
| Routes of administration |
• Intramuscular injection[1] • Subcutaneous injection[2] |
| Drug class | Progestin; Progestogen; Progestogen ester; Antigonadotropin |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability |
Oral: Very low (~3% in rats)[3] Intramuscular: 100% (in rats)[3] |
| Protein binding | Extensive (to albumin, not to CBG or (likely) SHBG)[1][4][5] |
| Metabolism | Reduction and hydroxylation (via CYP3A4, CYP3A5, CYP3A7) and conjugation (glucuronidation, sulfation, acetylation)[1] |
| Elimination half-life |
Non-pregnant: 7.8 days[6][7] Singlet: 16–17 days[1][8] Twins: 10 days[8] |
| Excretion |
Feces: 50%[1] Urine: 30%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard |
100.010.127 |
| Chemical and physical data | |
| Formula | C27H40O4 |
| Molar mass | 428.604 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hydroxyprogesterone caproate (OHPC), sold under the brand names Proluton and Makena among others, is a progestin medication which is used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological disorders.[1][7][8][9][10] It is also used in combination with an estrogen as a form of long-lasting injectable birth control.[11] It is not effective by mouth and must be given by injection into muscle, typically once per week.[1][3]
OHPC is generally well-tolerated and produces few side effects.[1] Injection site reactions such as pain and swelling are the most common side effect of OHPC.[1] The medication may increase the risk of gestational diabetes when used in pregnant women.[1][12] OHPC is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[12] It has some antimineralocorticoid activity and no other important hormonal activity.[13][14][15][16][17] The medication shows a number of differences from natural progesterone.[12][18]
OHPC was discovered in 1953 and was introduced for medical use in 1954 or 1955.[19][20] It was marketed in the United States under the brand name Delalutin and throughout Europe under the brand name Proluton.[19][21] The medication was discontinued in the United States in 1999.[22] However, OHPC was subsequently reintroduced in the United States under the brand name Makena for the treatment of preterm birth in 2011.[23] Due to a greatly increased price of the medication under this new formulation, a pricing controversy occurred in this country.[23] OHPC remains available at low cost from compounding pharmacies in the United States.[23][24]
Medical uses
Preterm birth
The use of OHPC in pregnancy to prevent preterm birth in women with a history of preterm delivery between 20 weeks and 36 weeks and 6 days is supported by the Society of Maternal Fetal Medicine Clinic Guidelines put out in May 2012 as Level I and III evidence, Level A recommendation.[25] Level I evidence refers to a properly powered randomized controlled trial, and level III evidence is support from expert opinion, while a Level A recommendation confers that the recommendation is made based on good and consistent scientific evidence. OHPC 250 mg IM weekly preferably starting at 16–20 weeks until 36 weeks is recommended. In these women, if the transvaginal ultrasound cervical length shortens to <25 mm at < 24 weeks, cervical cerclage may be offered. In the 2013 study the guideline recommendation is based on,[26] there was also a significant decrease of neonatal morbidity including lower rates of necrotizing enterocolitis (0 in the treatment group vs 4 in the control), intraventricular hemorrhage (4 in the treatment group compared with 8 in the control for a relative risk of 0.25), and need for supplemental oxygen (14% in the treatment group vs 24% in the placebo for a relative risk of 0.42). Furthermore, this study contained 463 women, 310 of whom received injection. Of these women, 9 had infants with congenital malformations (2%), but there was no consistent pattern and none involved internal organs.
OHPC is currently (as of June 2014) pregnancy category B, meaning there is no evidence of fetal risk with use of this medication during pregnancy. Although this is now the recommendation, this has not always been the case. A 2006 Cochrane Review concluded "...important maternal and infant outcomes have been poorly reported to date... information regarding the potential harms of progesterone therapy to prevent preterm birth is limited".[27] There was a similar conclusion from a review by Marc Keirse of Flinders University.[28] Three clinical studies in singleton pregnancies of 250 mg/week of intramuscular OHPC have all shown a trend for an increase in pregnancy loss due to miscarriage compared to placebo.[29][30][31] One of them, a large National Institutes of Health (NIH) study in 2003, looked at the effect of OHPC injections in women at risk for repeat premature birth and found that the treated group experienced premature birth in 37% versus 55% in the controls.[31] A follow-up study of the offspring showed no evidence that OHPC affected the children in the first years of life.[32] Based on these NIH data, OHPC was approved by the Food and Drug Administration (FDA) in 2011 as a medication to reduce the risk of premature birth in selected women at risk. (v.i.)
The FDA expressed concern about miscarriage at the 2006 advisory committee meeting; the committee voted unanimously that further study was needed to evaluate the potential association of OHPC with increased risk of second trimester miscarriage and stillbirth.[33] A toxicology study in rhesus monkeys resulted in the death of all rhesus fetuses exposed to 1 and 10 times the human dose equivalent of OHPC.[34] As of 2008, OHPC was a category D progestin according to the FDA (that is, there is evidence of fetal harm). There is speculation that the castor oil in the OHPC formulation may not be beneficial for pregnancy.[35][36] Of note, the above-mentioned NEJM study by Meirs et al. compares the effect of OHPC (with the castor oil component) to castor oil injection as the placebo.
A study published in February 2016 in The Lancet stated the below, amongst other findings:[37]
OPPTIMUM strongly suggests that the efficacy of progesterone in improving outcomes is either non-existent or weak. Given the heterogeneity of the preterm labour syndrome we cannot exclude benefit in specific phenotypic or genotypic subgroups of women at risk. However, the subgroups of women who might benefit do not appear to be easily identifiable by current selection strategies, including cervical length measurement and fibronectin testing.
Reassuringly, our study suggests that progesterone is safe for those who wish to take it for preterm birth prophylaxis. The overall rate of maternal or child adverse events was similar in the progesterone and placebo groups. There were few differences in the incidence of adverse secondary outcomes in the two groups, with the exception of a higher rate of renal, gastrointestinal, and respiratory complications in childhood in the progesterone groups. Importantly, the absolute rates of these complications was low. Follow-up of other babies exposed in utero to vaginal progesterone would be helpful in determining whether the increased rate of some renal, gastrointestinal, and respiratory complications is a real effect or a type I error.
The journal reviewer Richard Lehman, senior Research Fellow at the Department of Primary Health Care at the University of Oxford, made the following notable commentary on the OPPTIMUM study: "That's it. This story is ended, and nobody need ever use vaginal progesterone again to prevent preterm birth."[38]
Gynecological disorders
OHPC is used in the treatment of threatened miscarriage, gynecological disorders such as dysmenorrhea, premenstrual syndrome, fibrocystic breast disease, adenosis, and breast pain.[8] In addition, OHPC is used in the treatment of endometrial cancer and has been found to be significantly effective in extending life in both premenopausal and postmenopausal women with the disease.[39] The medication was used widely in the 1950s through the 1970s for such indications, but OHPC more recently has received the most attention in the prevention of preterm birth.[8]
Birth control
OHPC is available in combination with estradiol valerate as a once-monthly combined injectable contraceptive in a few countries.[11][40]
Other uses
OHPC has been used to treat benign prostatic hyperplasia in men, although evidence of effectiveness is marginal and uncertain.[41] The mechanism of action of OHPC in this use is suppression of testicular androgen production via suppression of luteinizing hormone secretion, which are the result of the progestogenic and antigonadotropic activity of OHPC.[41] However, symptoms of hypogonadism may develop when OHPC is used for this indication, with two-thirds of men reportedly experiencing impotence.[42]
OHPC has been used as a component of feminizing hormone therapy for transgender women.[43][44][45][46][47]
Cyclical intramuscular doses of 150 mg OHPC has been found in a study to be effective in the treatment of women with persistent, treatment-refractory acne, with 84% (64 of 76) responding to the treatment and experiencing a "good-to-excellent" improvement in symptoms.[48][49]
Available forms
OHPC is available alone in the form of ampoules and vials of 125 and 250 mg/mL oil solutions for intramuscular injection (brand names Proluton, Makena).[50][51] It is or was also available in combination with estradiol valerate in the form of ampoules and vials of 250 mg/mL / 5 mg/mL oil solutions for intramuscular injection (brand names Gravibinon and Chinese Injectable No. 1).[52][53][54][55] OHPC is or has been available in combination with estradiol benzoate in the form of ampoules of 125–250 mg / 10 mg oil solutions for intramuscular injection (brand name Primosiston) as well.[56][57][58][59][60]
Contraindications
Contraindications of OHPC include previous or current thrombosis or thromboembolic disease, known or suspected breast cancer, past or present history of other hormone-sensitive cancer, undiagnosed abnormal vaginal bleeding unrelated to pregnancy, cholestatic jaundice of pregnancy, liver tumors or active liver disease, and uncontrolled hypertension.[10] A few relative contraindications also exist for OHPC.[10]
Side effects
OHPC is generally well-tolerated and produces relatively few side effects.[1] Injection site reactions such as pain, soreness, swelling, itching, bruising, and lumps are the most common side effect of OHPC.[1] Side effects of OHPC that occur in greater than or equal to 2% of users include injection site pain (34.8%), injection site swelling (17.1%), urticaria (12.3%), pruritis (7.7%), injection site pruritus (5.8%), nausea (5.8%), injection site nodules (4.5%), and diarrhea (2.3%).[10] Numerically increased rates relative to controls of miscarriage (2.4% vs. 0%), stillbirth (2.0% vs. 1.3%), admission for preterm labor (16.0% vs. 13.8%), preeclampsia or gestational hypertension (8.8% vs. 4.6%), gestational diabetes (5.6% vs. 4.6%),[1][12] and oligohydramnios (3.6% vs. 1.3%) have been observed with OHPC in clinical trials in which it was given to pregnant women to prevent preterm birth.[10]
Overdose
There have been no reports of overdose of OHPC.[10] In the event of overdose, treatment should be based on symptoms.[10] OHPC has been studied in humans at high doses of 2,000 to 5,000 mg per week by intramuscular injection, without safety concerns.[6][17][61][62]
Interactions
OHPC is not likely to affect most cytochrome P450 enzymes at therapeutic concentrations.[10] Drug interaction studies have not been performed with OHPC.[10]
Pharmacology
Pharmacodynamics
OHPC has progestogenic activity, some antimineralocorticoid activity, and no other important hormonal activity.[13][7][14][15][61]
| Compound | hPR-A | hPR-B | rbPR | rbGR | rbER | |||
|---|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 100 | 100 | <1 | <1 | |||
| 17α-Hydroxyprogesterone | 1 | 1 | 3 | 1 | <1 | |||
| Hydroxyprogesterone caproate | 26 | 30 | 28 | 4 | <1 | |||
| Hydroxyprogesterone acetate | 38 | 46 | 115 | 3 | ND | |||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, dexamethasone for the GR, and estradiol for the ER. Sources:[63][64] | ||||||||
| Progestogen | Type | Class | TFD (14 days) | MDT (week) | OID (month) | POIC-D (2–3 months) | CIC-D (month) | Duration |
|---|---|---|---|---|---|---|---|---|
| Algestone acetophenide | Synthetic | Pregnane | ND | ND | ND | NA | 75–150 mg | ND |
| Gestonorone caproate | Synthetic | Norpregnane | ND | ND | ND | NA | NA | ND |
| Hydroxyprogesterone caproate | Synthetic | Pregnane | 250–500 mg | 25 mg | 250–500 mg | NA | 250–500 mg | 250 mg ≈ 10 days |
| Medroxyprogesterone acetate | Synthetic | Pregnane | 50–100 mg | ND | ND | 150 mg | 25 mg | 50 mg ≈ 14 days |
| Megestrol acetate | Synthetic | Pregnane | ND | ND | ND | NA | 25 mg | ND |
| Norethisterone enanthate | Synthetic | Estrane | ND | ND | ND | 200 mg | 50 mg | ND |
| Progesterone (oil soln.) | Bioidentical | Pregnane | 200 mg | ND | ND | NA | NA | 25 mg ≈ 2–3 days |
| Progesterone (cryst. susp.) | Bioidentical | Pregnane | 50–100 mg | ND | ND | NA | NA | 50 mg ≈ 14 days |
| Notes: All by intramuscular injection. Abbreviations: TFD = Endometrial transformation dose. MDT = Menstrual delay test dose (Greenblatt). OID = Ovulation-inhibiting dose (antigonadotropic effect; without an estrogen). POIC-D = Progestogen-only injectable contraceptive dose(s). CIC-D = Combined injectable contraceptive dose(s). Miscellaneous: Direct link to table. Sources:[65][66][54][67][55] | ||||||||
Progestogenic activity
OHPC, also known as 17α-hydroxyprogesterone caproate, is closer to progesterone in terms of structure and pharmacology than most other progestins, and is essentially a pure progestogen – that is, a selective agonist of the progesterone receptor (PR) with minimal or no other hormonal activity.[16][17] However, OHPC has improved pharmacokinetics compared to progesterone, namely a much longer duration with depot injection.[8][68][57][69]
Administered by intramuscular injection, the endometrial transformation dosage of OHPC per cycle is 250 to 500 mg, and the weekly substitution dosage of OHPC is 250 mg, while the effective dosage of OHPC in the menstrual delay test (Greenblatt) is 25 mg per week.[57][69][70] An effective ovulation-inhibiting dosage of OHPC is 500 mg once per month by intramuscular injection.[54][67][71] However, the dose of OHPC used in once-a-month combined injectable contraceptives is 250 mg, and this combination is effective for inhibition of ovulation similarly.[54][71] For comparison, the dose of medroxyprogesterone acetate (MPA; 6α-methyl-17α-hydroxyprogesterone acetate), a close analogue of OHPC, used by intramuscular injection in once-a-month combined injectable contraceptives is 25 mg.[54][67] It has also been said that given by intramuscular injection, 250 mg OHPC is equivalent to 50 mg medroxyprogesterone acetate.[72] It should be noted however that whereas the biological half-life of intramuscular OHPC in non-pregnant women is about 8 days,[6][7] the half-life of intramuscular medroxyprogesterone acetate in women is around 50 days.[73] OHPC is also less potent than the more closely related ester hydroxyprogesterone acetate (OHPA; 17α-hydroxyprogesterone acetate).[63]
17α-Hydroxyprogesterone (OHP) has weak progestogenic activity, but C17α esterification results in higher progestogenic activity.[60] Of a variety of different esters, the caproate (hexanoate) ester was found to have the strongest progestogenic activity, and this formed the basis for the development of OHPC, as well as other caproate progestogen esters such as gestonorone caproate.[60] OHPC is a much more potent progestogen than 17α-hydroxyprogesterone, but does not have as high of affinity for the PR as progesterone.[63] It has about 26% and 30% of the affinity of progesterone for the human PR-A and PR-B, respectively.[1][63] The medication was no more efficacious than progesterone in activating these receptors and eliciting associated gene expression in vitro.[1][63]
Antigonadotropic effects
Due to activation of the PR, OHPC has antigonadotropic effects, or produces suppression of the hypothalamic–pituitary–gonadal axis,[74][75] and can significantly suppress gonadotropin secretion and gonadal sex hormone production at sufficiently high doses.[76] One study found that 400 mg/week intramuscular OHPC suppressed luteinizing hormone by 24 to 30% and follicle-stimulating hormone levels by 19 to 55% in men.[77] In another study that used an unspecified dosage of intramuscular OHPC, testosterone secretion was assessed in a single man and was found to decrease from 4.2 mg/day to 2.0 mg/day (or by approximately 52%) by six weeks of treatment, whereas secretion of luteinizing hormone remained unchanged in the man.[78] The antigonadotropic effects of OHPC and hence its testosterone suppression are the basis of the use of OHPC in the treatment of benign prostatic hyperplasia in men. Suppression of luteinizing hormone levels by OHPC has also been observed in women.[79][80]
Glucocorticoid activity
OHPC is said not to have any glucocorticoid activity.[17] In accordance, OHPC has been found not to alter cortisol levels in humans even with very high doses by intramuscular injection.[6] This is of relevance because medications with significant glucocorticoid activity suppress cortisol levels due to increased negative feedback on the hypothalamic–pituitary–adrenal axis.[50][81][82] OHPC has been studied in humans at doses as high as 5,000 mg per week by intramuscular injection, with safety and without glucocorticoid effects observed.[6][62] The medication does interact with the glucocorticoid receptor however; it has about 4% of the affinity of dexamethasone for the rabbit glucocorticoid receptor.[1][63] But it acts as a partial agonist of the receptor and has no greater efficacy than progesterone in activating the receptor and eliciting associated gene expression in vitro.[1][63][83]
Other activities
As a pure progestogen, OHPC has no androgenic, antiandrogenic, estrogenic, or glucocorticoid activity.[16][17][84] The absence of androgenic and antiandrogenic activity with OHPC is in contrast to most other 17α-hydroxyprogesterone-derivative progestins.[68][84] Due to its lack of androgenic properties, similarly to progesterone, OHPC does not have any teratogenic effects on the fetus, making it safe for use during pregnancy.[17] Although OHPC has been described as a pure progestogen, there is evidence that it possesses some antimineralocorticoid activity, similarly to progesterone and 17α-hydroxyprogesterone.[14][15] Unlike progesterone, OHPC and its metabolites are not anticipated to interact with non-genomic receptors such as membrane progesterone receptors or the GABAA receptor.[18] In accordance, OHPC is not thought to possess the neurosteroid activities of progesterone or its associated sedative effects.[18]
In relation to cytochrome P450 enzymes, OHPC has no effect on CYP1A, CYP2D6, CYP2C9, or CYP3A4, but is a modest inducer of CYP2C19.[8]
Differences from progesterone
There are biological and pharmacological differences between progesterone and OHPC, which may have implications for the obstetrical use of OHPC.[12][18] These differences include the following:[12][18]
- Decreased myometrial activity with progesterone in vitro but no effect or increased myometrial activity with OHPC[85]
- Prevention of cervical ripening with progesterone but unknown effect with OHPC
- A non-significantly increased rate of stillbirth and miscarriages with OHPC (in one study)
- A possibly increased incidence of gestational diabetes with OHPC (increased in two studies, no difference in one study) but no such effect with progesterone
- A significantly increased risk of perinatal adverse effects such as fetal loss and preterm delivery in multiple gestations with OHPC (in two studies)
Differences in the metabolism of progesterone and OHPC and differences in the formation and activities of metabolites may be responsible for or involved in these observed biological and pharmacological differences.[18] Progesterone is metabolized by 5α- and 5β-reductases, 3α- and 3β-hydroxysteroid dehydrogenases, and 20α- and 20β-hydroxysteroid dehydrogenase in various tissues.[18][86] In target tissues, particularly the cervix and myometrium, these enzymes regulate local progesterone concentrations and can activate or inactivate progesterone signaling.[18] In addition, these enzymes catalyze the formation of metabolites of progesterone such as 5β-dihydroprogesterone and allopregnanolone, which signal through their own non-genomic receptors such as membrane progesterone receptors and the GABAA receptor and have their own important effects in pregnancy.[85][87][88] As examples, 5β-dihydroprogesterone has been found to play an important role in suppressing myometrial activity while allopregnanolone has potent sedative and anesthetic effects in the mother and especially the fetus and is involved in fetal nervous system development.[18][87][88][89][90] In contrast to progesterone, OHPC is not metabolized by traditional steroid-transforming enzymes and instead is metabolized exclusively via oxidation at the caproate side chain by cytochrome P450 enzymes.[18] As such, it is not thought to have the same tissue-specific activation and inactivation patterns that progesterone does nor the same non-genomic actions that progesterone and its metabolites possess.[18]
Further clinical research is anticipated to provide additional data to help clarify the issue of safety with OHPC.[12] In any case, it has been recommended by the American College of Obstetricians and Gynecologists that pregnant women treated with OHPC receive counseling about its risks and benefits and that such discussion be documented in the patient's chart.[12]
Pharmacokinetics
| Parameter | Singleton | Twin |
|---|---|---|
| Cmax (ng/mL) | 22.6 (15.8–27.4) | 17.3 (12–27) |
| Cmean(0–t) (ng/mL) | 16.8 (12.8–22.7) | 12.3 (8.4–18.7) |
| Ctrough (ng/mL) | 14.1 (10–18.1) | 11.2 (4.8–16.3) |
| AUC0–t (ng/mL/day) | 117.3 (89.9–159.1) | 86.1 (59–131) |
| t1/2 (days) | 16.2 (10.6–21.0) | 10 (6–16) |
| Tmax (days) | 1.0 (1–3) | 1.2 (1–2) |
| Vd/F (×103) (L) | 56 (25.2–69.6) | 16.9 (9.1–24.5) |
| Cl/F (×103) (L) | 2.1 (1.5–2.7) | 1.2 (0.9–1.7) |
| Footnotes: a = OHPC 250 mg once per week by intramuscular injection. Sources: [8][91][92] | ||
Absorption
In animals, the bioavailability of OHPC with intramuscular injection is nearly 100%, but its oral bioavailability is very low at less than 3%.[3] For this reason, oral administration of OHPC is unfeasible for medical use and the medication must instead be administered by intramuscular injection.[3] OHPC is one of the few clinically used progestins that is not active orally or used orally.[68] A depot effect occurs when OHPC is injected intramuscularly or subcutaneously, such that the medication has a prolonged duration of action.[2][8]
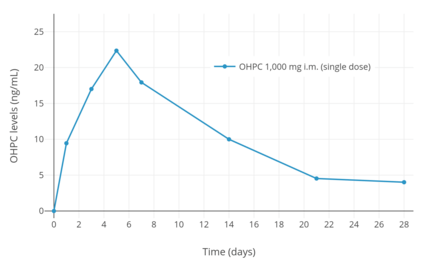
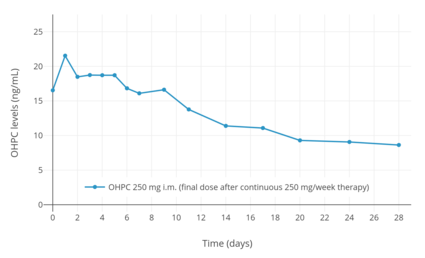
Following a single intramuscular injection of 1,000 mg OHPC in five women with endometrial cancer, peak levels of OHPC were 27.8 ± 5.3 ng/mL and the time to peak concentrations was 4.6 ± 1.7 (3–7) days.[6][93] Following 13 weeks of continuous administration of 1,000 mg OHPC per week, trough levels of OHPC were 60.0 ± 14 ng/mL.[6][93] The pharmacokinetic parameters of 250 mg OHPC once per week by intramuscular injection have also been studied in pregnant women with singleton and multiple (twin and triplet) gestation.[8][91][92] Steady state levels of the medication are achieved within 4 to 12 weeks of administration in pregnant women.[1]
Distribution
OHPC is extensively bound to plasma proteins, of which include albumin.[1] Unlike progesterone and 17α-hydroxyprogesterone, OHPC has very low affinity for corticosteroid-binding globulin (less than 0.01% of that of cortisol).[4] Progesterone and 17α-hydroxyprogesterone have low affinity for sex hormone-binding globulin, and for this reason, only a very small fraction of them (less than 0.5%) is bound to this protein in the circulation.[5]
Metabolism
OHPC appears to be metabolized primarily by the cytochrome P450 enzymes CYP3A4 and CYP3A5.[1] It may also be metabolized by CYP3A7 in fetal liver and the placenta.[1] Unlike progesterone, OHPC is not metabolized by traditional steroid-transforming enzymes and does not form similar metabolites.[18] The metabolism of OHPC is by reduction, hydroxylation, and conjugation, including glucuronidation, sulfation, and acetylation.[18] The caproate ester of OHPC is not cleaved during metabolism, so 17α-hydroxyprogesterone is not formed from OHPC.[18][63] As such, OHPC is not a prodrug of 17α-hydroxyprogesterone, nor of progesterone.[18][63]
OHPC has been found to have an elimination half-life of 7.8 days when given by intramuscular injection in an oil-based formulation to non-pregnant women.[6][7] Its total duration is said to be 10 to 14 days, which is much longer than the duration of intramuscularly administered progesterone in an oil formulation (2 to 3 days).[48] In pregnant women, the elimination half-life of OHPC appears to be longer, about 16 or 17 days.[1][8] However, in women pregnant with twins rather than a singlet, the elimination half-life of OHPC was found to be shorter than this, at 10 days.[8] OHPC has been detected in pregnant women up to 44 days after the last dose.[8]
Elimination
OHPC is eliminated 50% in feces and 30% in urine when given by intramuscular injection to pregnant women.[1] Both the free steroid and conjugates are excreted by these routes, with the conjugates more prominent in feces.[1]
Routes
OHPC has been found to possess similar pharmacokinetics, including peak levels, time to peak levels, area-under-the-curve levels (i.e., total exposure), and elimination half-life, with administration via intramuscular injection or subcutaneous autoinjection.[2] However, there was a higher incidence of injection site pain with subcutaneous autoinjection than with intramuscular injection (37.3% vs. 8.2%).[2]
Chemistry
OHPC, also known as 17α-hydroxyprogesterone caproate or as 17α-hydroxypregn-4-ene-3,20-dione 17α-hexanoate, is a synthetic pregnane steroid and a derivative of progesterone.[21][94] It is specifically a derivative of 17α-hydroxyprogesterone with a hexanoate (caproate) ester at the C17α position.[21][94] Analogues of OHPC include other 17α-hydroxyprogesterone derivatives such as algestone acetophenide (dihydroxyprogesterone acetophenide), chlormadinone acetate, cyproterone acetate, hydroxyprogesterone acetate, hydroxyprogesterone heptanoate, medroxyprogesterone acetate, and megestrol acetate, as well as the caproate esters chlormadinone caproate, gestonorone caproate (norhydroxyprogesterone caproate), medroxyprogesterone caproate, megestrol caproate, and methenmadinone caproate.[21][94]
Synthesis
Chemical syntheses of OHPC have been described.[95][96][60]
History
Along with hydroxyprogesterone acetate, OHPC was developed by Karl Junkmann of Schering AG in 1953 and was first reported by him in the medical literature in 1954.[19][97][98][99][100] It was reportedly first marketed in Japan in 1954 or 1955,[20] and was subsequently introduced as Delalutin in the United States in 1956.[8][101] After decades of use, Squibb, the manufacturer, voluntarily withdrew the brand; however, physicians continued to use OHPC "off-label".
Renewed interest on OHPC was sparked with a large NIH-sponsored study in 2003 that found that OHPC reduced the risk of premature birth in selected at-risk pregnant women.[31] With follow-up data showing no evidence of harmful effects on the offspring, the FDA approved the medication, as sponsored by KV Pharmaceutical as Makena, as an orphan drug in February 2011 to reduce the risk of premature birth in women prior to 37 weeks gestation with a single fetus who had at least one previous premature birth.[102] The medication is not effective in preventing premature birth in women with multiples. With the arrival of Makena as an orphan drug, the price of the medication was to increase from US$15 to US$1,500 per dose meaning a typical treatment would cost US$25,000 to US$30,000 – a pricing strategy that was strongly criticized. The FDA then announced that pharmacies could continue to compound the medication at their usual cost of approximately US$10 to US$20 per dose without fear of legal reprisals.,[24] and KV reduced its price to US$690 per dose.[103]
Society and culture
Generic names
Hydroxyprogesterone caproate is the generic name of OHPC and its INN, USAN, BANM, and JAN, while hydroxyprogesterone hexanoate was its former BANM.[21][40][94]
OHPC is often mislabeled as and confused with progesterone and 17α-hydroxyprogesterone.[104]
Brand names
OHPC is marketed throughout the world under a variety of brand names including Proluton, Proluton Depot, and Makena (US), among many others.[21][40][94] It was also formerly marketed under brand names including Delalutin, Prodrox, and Hylutin among others, but these formulations have since been discontinued.[21][94] It has been marketed under the brand names Gravibinon and Injectable No. 1 (or Chinese Injectable No. 1) in combination with estradiol valerate[52][53][54][55] and under the brand name Primosiston in combination with estradiol benzoate.[56][57][58][59][60]
Availability
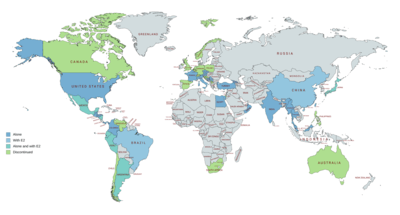
OHPC is marketed in the United States and throughout Europe, Asia, and Central and South America.[21][40][105][106] It is notably not available in Canada, the United Kingdom, New Zealand, or South Africa, and only veterinary formulations are available in Australia.[21][40][105][106] OHPC is also marketed in combination with estradiol valerate as a combined injectable contraceptive in a number of countries including in South America, Mexico, Japan, and China.[21][40][105][106] It has been marketed as an injectable preparation in combination with estradiol benzoate in some countries as well.[56][57][58][59][60]
Controversy
A 2011 decision by the FDA was going to result in driving "up the [U.S.] cost of an available medication from about US$300 to US$30,000 – about a 100-fold increase – with minimal added clinical benefit".[23] However, the FDA said that it would not go after compounding pharmacies that filled prescriptions, and KV Pharmaceutical announced a lower price.[23]
Research
OHPC was studied by Schering for use as a progestogen-only injectable contraceptive at a dose of 250 to 500 mg once a month by intramuscular injection but produced poor cycle control at these doses and was never marketed.[107][108]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 Deeks ED (2011). "17 α-Hydroxyprogesterone caproate (Makena™): in the prevention of preterm birth". Paediatr Drugs. 13 (5): 337–45. doi:10.2165/11208140-000000000-00000. PMID 21888448.
- 1 2 3 4 Krop J, Kramer WG (2017). "Comparative Bioavailability of Hydroxyprogesterone Caproate Administered via Intramuscular Injection or Subcutaneous Autoinjector in Healthy Postmenopausal Women: A Randomized, Parallel Group, Open-label Study". Clin Ther. 39 (12): 2345–2354. doi:10.1016/j.clinthera.2017.10.020. PMID 29191450.
- 1 2 3 4 5 Shaik, Imam H.; Bastian, Jaime R.; Zhao, Yang; Caritis, Steve N.; Venkataramanan, Raman (2015). "Route of administration and formulation dependent pharmacokinetics of 17-hydroxyprogesterone caproate in rats". Xenobiotica. 46 (2): 169–174. doi:10.3109/00498254.2015.1057547. ISSN 0049-8254. PMC 4809632.
- 1 2 Mickelson KE, Forsthoefel J, Westphal U (October 1981). "Steroid-protein interactions. Human corticosteroid binding globulin: some physicochemical properties and binding specificity". Biochemistry. 20 (21): 6211–8. PMID 7306509.
- 1 2 Dunn JF, Nisula BC, Rodbard D (July 1981). "Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 58–68. doi:10.1210/jcem-53-1-58. PMID 7195404.
- 1 2 3 4 5 6 7 8 9 Onsrud M, Paus E, Haug E, Kjørstad K (1985). "Intramuscular administration of hydroxyprogesterone caproate in patients with endometrial carcinoma. Pharmacokinetics and effects on adrenal function". Acta Obstet Gynecol Scand. 64 (6): 519–23. doi:10.3109/00016348509156732. PMID 2932883.
- 1 2 3 4 5 Hines M, Lyseng-Williamson KA, Deeks ED (March 2013). "17 α-hydroxyprogesterone caproate (Makena®): a guide to its use in the prevention of preterm birth". Clin Drug Investig. 33 (3): 223–7. doi:10.1007/s40261-013-0060-6. PMID 23413110.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Feghali, Maisa; Venkataramanan, Raman; Caritis, Steve (2014). "Prevention of preterm delivery with 17-hydroxyprogesterone caproate: Pharmacologic considerations". Seminars in Perinatology. 38 (8): 516–522. doi:10.1053/j.semperi.2014.08.013. ISSN 0146-0005. PMC 4253874. PMID 25256193.
- ↑ Manuck TA (2017). "17-alpha hydroxyprogesterone caproate for preterm birth prevention: Where have we been, how did we get here, and where are we going?". Semin. Perinatol. 41 (8): 461–467. doi:10.1053/j.semperi.2017.08.004. PMID 28947068.
- 1 2 3 4 5 6 7 8 9 https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021945s012lbl.pdf
- 1 2 Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- 1 2 3 4 5 6 7 8 Romero R, Stanczyk FZ (2013). "Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice". Am. J. Obstet. Gynecol. 208 (6): 421–6. doi:10.1016/j.ajog.2013.04.027. PMC 4120746. PMID 23643669.
- 1 2 J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 95–. ISBN 978-94-009-8195-9.
- 1 2 3 Ralph I. Dorfman (5 December 2016). Steroidal Activity in Experimental Animals and Man. Elsevier Science. pp. 398–. ISBN 978-1-4832-7299-3.
Intramuscular administration of 17α-hydroxyprogesterone caproate produced signs and symptoms of adrenal insufficiency in Addisonians maintained on cortisol and 9α-fluorocortisol (Melby, 1961) and thereby showed properties similar to progesterone and 17α-hydroxyprogesterone. However, further tests will be required to eludicate its pharmacodynamics properties. Contrastingly, there was no evidence for salt dissipation with the test of a smaller dose of the steroid to normal subjects (Landau et al., 1958).
- 1 2 3 Sammour MB, El-Kabarity H, Khalifa AS (1975). "Progesterone therapy in pre-eclamptic toxaemia". Acta Obstet Gynecol Scand. 54 (3): 195–202. doi:10.3109/00016347509157760. PMID 1163210.
Melby (14) found that when progesterone was administered to patients suffering from the syndrome of idiopathic oedema, they experienced a diuresis, with a high excretion of sodium and water within 24 hours after a single injection of 500 mg of 17-α-hydroxyprogesterone caproate.
- 1 2 3 Geller, Jack (1965). "Treatment of Benign Prostatic Hypertrophy With Hydroxyprogesterone Caproate". JAMA. 193 (2): 121. doi:10.1001/jama.1965.03090020035009. ISSN 0098-7484. PMID 14304354.
- 1 2 3 4 5 6 Meis PJ (2005). "17 hydroxyprogesterone for the prevention of preterm delivery". Obstet Gynecol. 105 (5 Pt 1): 1128–35. doi:10.1097/01.AOG.0000160432.95395.8f. PMID 15863556.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Byrns MC (2014). "Regulation of progesterone signaling during pregnancy: implications for the use of progestins for the prevention of preterm birth". J. Steroid Biochem. Mol. Biol. 139: 173–81. doi:10.1016/j.jsbmb.2013.01.015. PMID 23410596.
- 1 2 3 M. Edward Davis. M. Edward Davis Reprints. p. 406.
Chemically pure progesterone was the only substance with progestational properties in general use which could be administered parenterally until Junkmann (1) developed in 1953, 17-alpha-hydroxyprogesterone acetate and 17-alpha-hydroxyprogesterone caproate.
- 1 2 International Agency for Research on Cancer (1979). Sex Hormones (II). International Agency for Research on Cancer. p. 401. ISBN 978-92-832-1221-8.
17α-Hydroxyprogesterone caproate was first marketed commercially in Japan in 1954-1955.
- 1 2 3 4 5 6 7 8 9 10 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1.
- ↑ Kim S. "The Orphan Drug Act: How the FDA Unlawfully Usurped Market Exclusivity". Heinonline.
- 1 2 3 4 5 Armstrong J (May 2011). "Unintended consequences — the cost of preventing preterm births after FDA approval of a branded version of 17OHP". N. Engl. J. Med. 364 (18): 1689–91. doi:10.1056/NEJMp1102796. PMID 21410391.
- 1 2 "Archived copy". Archived from the original on 2011-12-14. Retrieved 2011-03-30.
- ↑ SMFM Clinical Guideline: Progesterone and preterm birth prevention: translating clinical trials data into clinical practice, AJOG May 2012
- ↑ Meirs et al. NEJM 2003
- ↑ Dodd JM, Flenady V, Cincotta R, Crowther CA; The Cochrane Database of Systematic Reviews 2006 Issue 1
- ↑ Keirse, MJNC; Progesterone (2004). "déjà vu" or "still to be seen"?". Birth. 31: 3.
- ↑ Johnson, JWC; Austin, KL; Jones, GS; Davis, GH; King, TM (1975). "Efficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of premature labor". NEJM. 293 (14): 675–680. doi:10.1056/nejm197510022931401.
- ↑ Yemini, M; Borenstein, R; Dreazen; et al. (1985). "Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate". Am J Obstet Gynecol. 151 (5): 574–7. doi:10.1016/0002-9378(85)90141-3.
- 1 2 3 Meis PJ; Klebanoff M; Thom E; Dombrowski MP; Sibai B; Moawad AH; Spong CY; Hauth JC; Miodovnik M; Varner MW; Leveno KJ; Caritis SN; Iams JD; Wapner RJ; Conway D; O'Sullivan MJ; Carpenter M; Mercer B; Ramin SM; Thorp JM; Peaceman AM; Gabbe S; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. (2003). "Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate". N Engl J Med. 348 (24): 2379–85. doi:10.1056/NEJMoa035140. PMID 12802023.
- ↑ Northen AT; Norman GS; Anderson K; Moseley L; Divito M; Cotroneo M; Swain M; Bousleiman S; Johnson F; Dorman K; Milluzzi C; Tillinghast JA; Kerr M; Mallett G; Thom E; Pagliaro S; Anderson GD; National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. (2007). "Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo". Obstet. Gynecol. 110 (4): 865–72. doi:10.1097/01.AOG.0000281348.51499.bc. PMID 17906021.
- ↑ Advisory Committees: CDER 2006 Meeting Documents
- ↑ Hendrix AG, et al. Embriotoxicity of sex steroidal hormones in nonhuman primates: II. Hydroxyprogesterone caproate, estradiol valerate. Teratology 1987 February. 35 (1): 129.
- ↑ Duke University Medical Center, New England Journal of Medicine, correspondence, vol 349.
- ↑ Hauth, JC; Gilstrap, LC; Brekken, AL; Hauth, JM (1983). "The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population". Am J Obstet Gynecol. 146 (2): 187.
- ↑ Norman, Jane Elizabeth; Marlow, Neil; Messow, Claudia-Martina; Shennan, Andrew; Bennett, Phillip R; Thornton, Steven; Robson, Stephen C; McConnachie, Alex; Petrou, Stavros (2016-05-21). "Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial". The Lancet. 387 (10033): 2106–2116. doi:10.1016/S0140-6736(16)00350-0. ISSN 0140-6736. PMID 26921136.
- ↑ "BMJ Blogs: The BMJ » Blog Archive » Richard Lehman's journal review—23 May 2016". blogs.bmj.com. Retrieved 2016-05-25.
- ↑ Reifenstein, Edward C. (1971). "Hydroxyprogesterone caproate therapy in advanced endometrial cancer". Cancer. 27 (3): 485–502. doi:10.1002/1097-0142(197103)27:3<485::AID-CNCR2820270302>3.0.CO;2-1. ISSN 0008-543X.
- 1 2 3 4 5 6 https://www.drugs.com/international/hydroxyprogesterone.html
- 1 2 Benign Prostatic Hypertrophy. Springer Science & Business Media. 6 December 2012. pp. 266–. ISBN 978-1-4612-5476-8.
- ↑ Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 289–. ISBN 978-0-08-093292-7.
- ↑ Gianna E. Israel; Donald E. Tarver; Joy Diane Shaffer (1 March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 58–. ISBN 978-1-56639-852-7.
- ↑ Richard Ekins; Dave King (23 October 2006). The Transgender Phenomenon. SAGE Publications. pp. 48–. ISBN 978-1-84787-726-0.
- ↑ Richard K. Adler; Sandy Hirsch; Michelle Mordaunt (1 May 2012). Voice and Communication Therapy for The Transgender/Transsexual Client: A Comprehensive Clinical Guide. Plural Publishing. pp. 486–. ISBN 978-1-59756-631-5.
- ↑ Masumori, Naoya (2012). "Status of sex reassignment surgery for gender identity disorder in Japan". International Journal of Urology. 19 (5): 402–414. doi:10.1111/j.1442-2042.2012.02975.x. ISSN 0919-8172.
- ↑ Sharula, Chekir C, Emi Y, Arai F, Kikuchi Y, Sasaki A, Matsuda M, Shimizu K, Tabuchi K, Kamada Y, Hiramatsu Y, Nakatsuka M (2012). "Altered arterial stiffness in male-to-female transsexuals undergoing hormonal treatment". J. Obstet. Gynaecol. Res. 38 (6): 932–40. doi:10.1111/j.1447-0756.2011.01815.x. PMID 22487218.
- 1 2 Baker, Kenneth C. (1958). "Treatment of Persistent Acne in Women with 17 Alpha Hydroxyprogesterone Caproate (Delalutin)". Journal of Investigative Dermatology. 31 (5): 247–250. doi:10.1038/jid.1958.114. ISSN 0022-202X.
- ↑ Antibiotic Medicine and Clinical Therapy. 1959. p. 249.
- 1 2 Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 757–759, 2168. ISBN 978-0-7817-1750-2.
- ↑ Sandeep Nema; John D. Ludwig (19 April 2016). Pharmaceutical Dosage Forms - Parenteral Medications, Third Edition: Volume 1: Formulation and Packaging. CRC Press. pp. 161–. ISBN 978-1-4200-8644-7.
- 1 2 Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 561–. ISBN 978-3-7692-2114-5.
- 1 2 Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- 1 2 3 4 5 6 Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- 1 2 3 Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- 1 2 3 Freimut A. Leidenberger (17 April 2013). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 533–. ISBN 978-3-662-08110-5.
- 1 2 3 4 5 Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214, 255. ISBN 978-3-662-00942-0.
- 1 2 3 Heinrich Kahr (8 March 2013). Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. pp. 22–. ISBN 978-3-7091-5694-0.
- 1 2 3 Walther Kern; H. Auterhoff; F. Neuwald; W. Schmid (9 March 2013). Hagers Handbuch der Pharmazeutischen Praxis: Für Apotheker, Arzneimittelhersteller, Drogisten, Ärzte und Medizinalbeamte. Springer-Verlag. pp. 1163–. ISBN 978-3-642-49759-9.
- 1 2 3 4 5 6 Die Gestagene. Springer-Verlag. 27 November 2013. pp. 6, 1045–. ISBN 978-3-642-99941-3.
- 1 2 Hall NR (June 2011). "What agent should be used to prevent recurrent preterm birth: 17-P or natural progesterone?". Obstet. Gynecol. Clin. North Am. 38 (2): 235–46, ix–x. doi:10.1016/j.ogc.2011.02.014. PMID 21575799.
- 1 2 Varga A, Henriksen E. Clinical and Histopathologic Evaluation of the Effect of 17-alpha-Hydroxyprogesterone-17-n-caproate on Endometrial Carcinoma. Obstetrics & Gynecology. December 1961. Volume 18. Issue 6. pp. 658-672.
- 1 2 3 4 5 6 7 8 9 Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- ↑ Terenius L (June 1974). "Affinities of progestogen and estrogen receptors in rabbit uterus for synthetic progestogens". Steroids. 23 (6): 90919. doi:10.1016/0039-128X(74)90063-4. PMID 4134774.
- ↑ Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ↑ Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- 1 2 3 Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- 1 2 3 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ↑ Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- 1 2 Siegel I (June 1963). "Conception control by long-acting progestogens: preliminary report". Obstet Gynecol. 21: 666–8. doi:10.1097/00006250-196306000-00003. PMID 13992789.
- ↑ Kistner, Robert W. (1960). "The Use of Progestational Agents in Obstetrics and Gynecology". Clinical Obstetrics and Gynecology. 3 (4): 1047–1067. doi:10.1097/00003081-196003040-00019. ISSN 0009-9201.
50 mg of [medroxyprogesterone acetate], intramuscularly, is equivalent to 250 mg [hydroxyprogesterone caproate]
- ↑ "Depo_Provera" (PDF). FDA. 2016. Retrieved 31 March 2018.
- ↑ Yang D, Zhu RL (1994). "[Changes in reproductive hormones levels in the treatment of endometrial precancerous lesion with hydroxyprogesterone caproate]". Zhonghua Fu Chan Ke Za Zhi (in Chinese). 29 (4): 205–6, 251. PMID 8082440.
In this paper, 14 cases of precancerous lesion of endometrium were treated with hydroxyprogesterone caproate and a series of hormone determination was analysed before and after treatment. Results showed that LH and LH/FSH were dramatically decreased. (LH P < 0.05, LH/FSH P < 0.01).
- ↑ Benign Prostatic Hypertrophy. Springer Science & Business Media. 6 December 2012. pp. 266–. ISBN 978-1-4612-5476-8.
Since the initial report by Geller and associates28 on the use of hydroxyprogesterone caproate in the treatment of BPH, a variety of progestins have been studied in the medical management of this disease: hydroxyprogesterone caproate, chlormadinone acetate,27 and medrogestone (6-methyl-6-dehydro-17-methylprogesterone).50 These drugs should have a beneficial effect in BPH as they inhibit testicular function by suppressing serum LH and have no intrinsic estrogenic or androgenic activity.
- ↑ J.E. Castro (9 March 2013). The Treatment of Prostatic Hypertrophy and Neoplasia. Springer Science & Business Media. pp. 39–. ISBN 978-94-015-7190-6.
Geller has also demonstrated significant decreases in plasma or urine testosterone glucuronide levels following the administration of three other anti-androgens. These include Delalutin [hydroxyprogesterone caproate], chlormadinone acetate, and PH-218. It would appear that decreased androgen production is a property shared by all anti-androgens to date.
- ↑ Meiraz D, Margolin Y, Lev-Ran A, Lazebnik J (1977). "Treatment of benign prostatic hyperplasia with hydroxyprogesterone-caproate: placebo-controlled study". Urology. 9 (2): 144–8. doi:10.1016/0090-4295(77)90184-4. PMID 65818.
- ↑ Geller J, Bora R, Roberts T, Newman H, Lin A, Silva R (1965). "Treatment of benign prostatic hypertrophy with hydroxyprogesterone caproate: effect on clinical symptoms, morphology, and endocrine function". JAMA. 193: 121–8. doi:10.1001/jama.1965.03090020035009. PMID 14304354.
- ↑ Sherman AI, Woolf RB (February 1959). "An endocrine basis for endometrial carcinoma". Am. J. Obstet. Gynecol. 77 (2): 233–42. doi:10.1016/0002-9378(59)90223-6. PMID 13617315.
- ↑ Moe N (1972). "Short-term progestogen treatment of endometrial carcinoma. Histological, histochemical and hormonal studies". Acta Obstet Gynecol Scand. 51 (1): 55–62. doi:10.3109/00016347209154968. PMID 4261828.
- ↑ Eliza B. Geer (1 December 2016). The Hypothalamic-Pituitary-Adrenal Axis in Health and Disease: Cushing’s Syndrome and Beyond. Springer. pp. 28–. ISBN 978-3-319-45950-9.
- ↑ Diane S. Aschenbrenner; Samantha J. Venable (2009). Drug Therapy in Nursing. Lippincott Williams & Wilkins. pp. 674–. ISBN 978-0-7817-6587-9.
- ↑ Gerber AN, Masuno K, Diamond MI (March 2009). "Discovery of selective glucocorticoid receptor modulators by multiplexed reporter screening". Proc. Natl. Acad. Sci. U.S.A. 106 (12): 4929–34. doi:10.1073/pnas.0812308106. PMC 2660744. PMID 19255438.
- 1 2 Bardin CW, Brown T, Isomaa VV, Jänne OA (1983). "Progestins can mimic, inhibit and potentiate the actions of androgens". Pharmacol. Ther. 23 (3): 443–59. PMID 6371845.
- 1 2 Anderson L, Martin W, Higgins C, Nelson SM, Norman JE (2009). "The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy". Reprod Sci. 16 (11): 1052–61. doi:10.1177/1933719109340926. PMID 19602723.
- ↑ Perry T. Cupps (20 February 1991). Reproduction in Domestic Animals. Elsevier. pp. 101–. ISBN 978-0-08-057109-6.
- 1 2 Gellersen B, Fernandes MS, Brosens JJ (2009). "Non-genomic progesterone actions in female reproduction". Hum. Reprod. Update. 15 (1): 119–38. doi:10.1093/humupd/dmn044. PMID 18936037.
- 1 2 Schumacher M, Mattern C, Ghoumari A, Oudinet JP, Liere P, Labombarda F, Sitruk-Ware R, De Nicola AF, Guennoun R (2014). "Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors". Prog. Neurobiol. 113: 6–39. doi:10.1016/j.pneurobio.2013.09.004. PMID 24172649.
- ↑ Mellor DJ, Diesch TJ, Gunn AJ, Bennet L (2005). "The importance of 'awareness' for understanding fetal pain". Brain Res. Brain Res. Rev. 49 (3): 455–71. doi:10.1016/j.brainresrev.2005.01.006. PMID 16269314.
- ↑ Lagercrantz H, Changeux JP (2009). "The emergence of human consciousness: from fetal to neonatal life". Pediatr. Res. 65 (3): 255–60. doi:10.1203/PDR.0b013e3181973b0d. PMID 19092726.
[...] the fetus is sedated by the low oxygen tension of the fetal blood and the neurosteroid anesthetics pregnanolone and the sleep-inducing prostaglandin D2 provided by the placenta (36).
- 1 2 3 Caritis SN, Sharma S, Venkataramanan R, Hankins GD, Miodovnik M, Hebert MF, Umans JG, Benedetti T, Mattison D, Zajicek A, Fischer D, Jackson A (November 2012). "Pharmacology and placental transport of 17-hydroxyprogesterone caproate in singleton gestation". Am. J. Obstet. Gynecol. 207 (5): 398.e1–8. doi:10.1016/j.ajog.2012.08.015. PMC 3586341. PMID 22967833.
- 1 2 Caritis SN, Sharma S, Venkataramanan R, Rouse DJ, Peaceman AM, Sciscione A, Spong CY, Varner MW, Malone FD, Iams JD, Mercer BM, Thorp JM, Sorokin Y, Carpenter M, Lo J, Ramin S, Harper M (July 2011). "Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation". Am. J. Obstet. Gynecol. 205 (1): 40.e1–8. doi:10.1016/j.ajog.2011.03.028. PMC 3165062. PMID 21620357.
- 1 2 https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021945s000lbl.pdf
- 1 2 3 4 5 6 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 664–. ISBN 978-1-4757-2085-3.
- ↑ Jürgen Engel; Axel Kleemann; Bernhard Kutscher; Dietmar Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 677–679. ISBN 978-3-13-179275-4.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1865–1866. ISBN 978-0-8155-1856-3.
- ↑ WIED GL, DAVIS ME (1958). "Comparative activity of progestational agents on the human endometrium and vaginal epithelium of surgical castrates". Ann. N. Y. Acad. Sci. 71 (5): 599–616. doi:10.1111/j.1749-6632.1958.tb46791.x. PMID 13583817.
In the group of new parenteral progestational agents, three substances developed by Karl Junkmann1,2 are the most outstanding and interesting: 17a-hydroxyprogesterone caproate and 17a-hydroxyprogesterone acetate, introduced in 1953, and the most potent of all new parenteral progestational agents, 17-ethynyl-19-nortestosterone enanthate, introduced in 1956.
- ↑ ACRH. U.S. Dept. of Energy. 1960. p. 71.
[The] minimal activity [of 17(a)-hydroxyprogesterone] is magnified to an unexpected degree by the esterification of this steroid with caproic acid to produce 17(a)-hydroxyprogesterone-17-n-caproate, first reported by Karl Junkmann in 1954.6,7
- ↑ Ralph Isadore Dorfman (1966). Methods in Hormone Research. Academic Press. p. 86.
Junkmann (1954) reported that the acetate, butyrate, and caproate forms had both increased and prolonged activity, [...]
- ↑ Norman Applezweig (1962). Steroid Drugs. Blakiston Division, McGraw-Hill. pp. 101–102.
Junkmann of Schering, AG., however, was able to show that long chain esters of 17a-hydroxyprogesterones such as the 17a-caproate produced powerful long-acting progestational effect. This compound is marketed in the United States as Delalutin by Squibb, and has been heavily used for the treatment of habitual abortion.
- ↑ New and Nonofficial Drugs. Lippincott. 1958. p. 662.
Supplied by.—E. R. Squibb & Sons (Delalutin). Year of introduction: 1956.
- ↑ FDA press release regarding Makena approval
- ↑ "Price of preterm birth medicine cut". Boston.com. Associated Press. April 2, 2011. Retrieved April 2, 2011.
- ↑ O'Brien JM (September 2013). "Medication safety is still an issue in obstetrics 50 years after the Kefauver-Harris amendments: the case of progestogens". Ultrasound Obstet Gynecol. 42 (3): 247–53. doi:10.1002/uog.12456. PMID 23495199.
- 1 2 3 http://www.micromedexsolutions.com/micromedex2/
- 1 2 3 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. pp. 2110–2111. ISBN 978-0-85369-840-1.
- ↑ Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289
- ↑ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
Further reading
- Meis PJ (May 2005). "17 hydroxyprogesterone for the prevention of preterm delivery". Obstet Gynecol. 105 (5 Pt 1): 1128–35. doi:10.1097/01.AOG.0000160432.95395.8f. PMID 15863556.
- Facchinetti F, Vaccaro V (October 2009). "Pharmacological use of progesterone and 17-alpha-hydroxyprogesterone caproate in the prevention of preterm delivery". Minerva Ginecol. 61 (5): 401–9. PMID 19749671.
- Deeks ED (October 2011). "17 α-Hydroxyprogesterone caproate (Makena™): in the prevention of preterm birth". Paediatr Drugs. 13 (5): 337–45. doi:10.2165/11208140-000000000-00000. PMID 21888448.
- Merlob P, Stahl B, Klinger G (January 2012). "17α Hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth". Reprod. Toxicol. 33 (1): 15–9. doi:10.1016/j.reprotox.2011.10.017. PMID 22120850.
- O'Brien JM (October 2012). "The safety of progesterone and 17-hydroxyprogesterone caproate administration for the prevention of preterm birth: an evidence-based assessment". Am J Perinatol. 29 (9): 665–72. doi:10.1055/s-0032-1316444. PMID 22773279.
- Romero R, Stanczyk FZ (June 2013). "Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice". Am. J. Obstet. Gynecol. 208 (6): 421–6. doi:10.1016/j.ajog.2013.04.027. PMC 4120746. PMID 23643669.
- Feghali M, Venkataramanan R, Caritis S (December 2014). "Prevention of preterm delivery with 17-hydroxyprogesterone caproate: pharmacologic considerations". Semin. Perinatol. 38 (8): 516–22. doi:10.1053/j.semperi.2014.08.013. PMC 4253874. PMID 25256193.
- Saccone G, Suhag A, Berghella V (July 2015). "17-alpha-hydroxyprogesterone caproate for maintenance tocolysis: a systematic review and metaanalysis of randomized trials". Am. J. Obstet. Gynecol. 213 (1): 16–22. doi:10.1016/j.ajog.2015.01.054. PMID 25659469.
- O'Brien JM, Lewis DF (January 2016). "Prevention of preterm birth with vaginal progesterone or 17-alpha-hydroxyprogesterone caproate: a critical examination of efficacy and safety". Am. J. Obstet. Gynecol. 214 (1): 45–56. doi:10.1016/j.ajog.2015.10.934. PMID 26558340.
- Caritis SN, Feghali MN, Grobman WA, Rouse DJ (August 2016). "What we have learned about the role of 17-alpha-hydroxyprogesterone caproate in the prevention of preterm birth". Semin. Perinatol. 40 (5): 273–80. doi:10.1053/j.semperi.2016.03.002. PMC 4983195. PMID 27105940.
- Saccone G, Khalifeh A, Elimian A, Bahrami E, Chaman-Ara K, Bahrami MA, Berghella V (March 2017). "Vaginal progesterone vs intramuscular 17α-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta-analysis of randomized controlled trials". Ultrasound Obstet Gynecol. 49 (3): 315–321. doi:10.1002/uog.17245. PMID 27546354.
- Oler E, Eke AC, Hesson A (July 2017). "Meta-analysis of randomized controlled trials comparing 17α-hydroxyprogesterone caproate and vaginal progesterone for the prevention of recurrent spontaneous preterm delivery". Int J Gynaecol Obstet. 138 (1): 12–16. doi:10.1002/ijgo.12166. PMID 28369874.
- Manuck TA (December 2017). "17-alpha hydroxyprogesterone caproate for preterm birth prevention: Where have we been, how did we get here, and where are we going?". Semin. Perinatol. 41 (8): 461–467. doi:10.1053/j.semperi.2017.08.004. PMID 28947068.