Baicalein
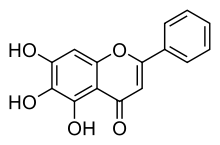
Baicalein (5,6,7-trihydroxyflavone) is a flavone, a type of flavonoid, originally isolated from the roots of Scutellaria baicalensis and Scutellaria lateriflora. It is also reported in Oroxylum indicum (Indian trumpetflower) and Thyme.[1] It is the aglycone of baicalin. Baicalein is one of the active ingredients of Sho-Saiko-To, a Chinese herbal supplement believed to enhance liver health.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5,6,7-Trihydroxy-2-phenyl-chromen-4-one | |
| Other names
5,6,7-Trihydroxyflavone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.911 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Baicalein, along with its analogue baicalin, is a positive allosteric modulator of the benzodiazepine site and/or a non-benzodiazepine site of the GABAA receptor.[2][3][4][5][6][7] It displays subtype selectivity for α2 and α3 subunit-containing GABAA receptors.[8] In accordance, baicalein shows anxiolytic effects in mice without incidence of sedation or myorelaxation.[7][8][9] It is thought that baicalein, along with other flavonoids, may underlie the anxiolytic effects of S. baicalensis and S. lateriflora.[10][11] Baicalein is also an antagonist of the estrogen receptor, or an antiestrogen.[11]
The flavonoid has been shown to inhibit certain types of lipoxygenases[12] and act as an anti-inflammatory agent.[13] It has antiproliferative effects on ET-1-induced proliferation of pulmonary artery smooth muscle cell proliferation via inhibition of TRPC1 channel expression.[14] Possible antidepressant effects have also been attributed to baicalein in animal research.[15]
Baicalein is an inhibitor of CYP2C9,[16] an enzyme of the cytochrome P450 system that metabolizes drugs in the body.
A derivative of baicalin is a known prolyl endopeptidase inhibitor.[17]
Baicalein has been shown to inhibit Staphylococcus aureus biofilm formation and the quorum sensing system in vitro.[18]
It has also been shown to be effective in vitro against all forms of Borrelia burgdorferi and Borrelia garinii.[19]
References
- Matsumoto, Takumi (2008). Phytochemistry Research Progress. ISBN 9781604562323.
- Wang H, Hui KM, Xu S, Chen Y, Wong JT, Xue H (2002). "Two flavones from Scutellaria baicalensis Georgi and their binding affinities to the benzodiazepine site of the GABAA receptor complex". Pharmazie. 57 (12): 857–8. PMID 12561253.
- Hui KM, Wang XH, Xue H (2000). "Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site". Planta Med. 66 (1): 91–3. doi:10.1055/s-0029-1243121. PMID 10705749.
- Zhang SQ, Obregon D, Ehrhart J, Deng J, Tian J, Hou H, Giunta B, Sawmiller D, Tan J (2013). "Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model". J. Neurosci. Res. 91 (9): 1239–46. doi:10.1002/jnr.23244. PMC 3810722. PMID 23686791.
- Liao JF, Wang HH, Chen MC, Chen CC, Chen CF (1998). "Benzodiazepine binding site-interactive flavones from Scutellaria baicalensis root". Planta Med. 64 (6): 571–2. doi:10.1055/s-2006-957517. PMID 9776664.
- Edwin Lowell Cooper; Nobuo Yamaguchi (1 January 2004). Complementary and Alternative Approaches to Biomedicine. Springer Science & Business Media. pp. 188–. ISBN 978-0-306-48288-5.
- de Carvalho RS, Duarte FS, de Lima TC (2011). "Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice". Behav. Brain Res. 221 (1): 75–82. doi:10.1016/j.bbr.2011.02.038. PMID 21377498.
- Wang F, Xu Z, Ren L, Tsang SY, Xue H (2008). "GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin". Neuropharmacology. 55 (7): 1231–7. doi:10.1016/j.neuropharm.2008.07.040. PMID 18723037.
- Liao JF, Hung WY, Chen CF (2003). "Anxiolytic-like effects of baicalein and baicalin in the Vogel conflict test in mice". Eur. J. Pharmacol. 464 (2–3): 141–6. doi:10.1016/s0014-2999(03)01422-5. PMID 12620506.
- Awad R, Arnason JT, Trudeau V, Bergeron C, Budzinski JW, Foster BC, Merali Z (2003). "Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): a medicinal plant with anxiolytic properties". Phytomedicine. 10 (8): 640–9. doi:10.1078/0944-7113-00374. PMID 14692724.
- Stefanie Schwartz (9 January 2008). Psychoactive Herbs in Veterinary Behavior Medicine. John Wiley & Sons. pp. 139–. ISBN 978-0-470-34434-7.
- Deschamps JD, Kenyon VA, Holman TR (June 2006). "Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases". Bioorg. Med. Chem. 14 (12): 4295–301. doi:10.1016/j.bmc.2006.01.057. PMID 16500106.
- Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS (2007). "Baicalein inhibits IL-1β- and TNF-α-induced inflammatory cytokine production from human mast cells via regulation of the NF-κB pathway". Clin Mol Allergy. 5 (1): 5. doi:10.1186/1476-7961-5-5. PMC 2206049. PMID 18039391.
- Lin Y-L, Lin R-J, Shen K-P, Dai Z-K, Chen I-J, Wu J-R, Wu B-N (2011). "Baicalein, isolated from Scutellaria baicalensis, protects against endothelin-1-induced pulmonary artery smooth muscle cell proliferation via inhibition of TRPC1 channel expression". Journal of Ethnopharmacology. 138 (2): 373–381. doi:10.1016/j.jep.2011.09.014. PMID 21963569.
- Xiong Z, Jiang B, Wu PF, et al. (2011). "Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade". Biol. Pharm. Bull. 34 (2): 253–9. doi:10.1248/bpb.34.253. PMID 21415537.
- Si D, Wang Y, Zhou Y-H, Guo Y, Wang J, Zhou H, Li Z-S, Fawcett JP (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols" (PDF). Drug Metabolism and Disposition. 37 (3): 629–34. doi:10.1124/dmd.108.023416. PMID 19074529.
- Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E (Aug 2008). "Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor". Bioorganic and Medicinal Chemistry. 16 (15): 7516–24. doi:10.1016/j.bmc.2008.04.067. PMID 18650094.
- Chen Y, Liu T, Wang K, Hou C, Cai S, Huang Y, Du Z, Huang H, Kong J, Chen Y (April 2016). "Baicalein Inhibits Staphylococcus aureus Biofilm Formation and the Quorum Sensing System In Vitro". PLOS ONE. 11 (4): e0153468. doi:10.1371/journal.pone.0153468. PMC 4851419. PMID 27128436.
- Goc, A.; Niedzwiecki, A.; Rath, M. (December 2015). "In vitro evaluation of antibacterial activity of phytochemicals and micronutrients against Borrelia burgdorferi and Borrelia garinii". Journal of Applied Microbiology. 119 (6): 1561–1572. doi:10.1111/jam.12970. ISSN 1365-2672. PMC 4738477. PMID 26457476.