Rifaximin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xifaxan, Xifaxanta, Normix, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604027 |
| Pregnancy category |
|
| Routes of administration | by mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | < 0.4% |
| Metabolism | Hepatic |
| Elimination half-life | 6 hours |
| Excretion | Fecal (97%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.111.624 |
| Chemical and physical data | |
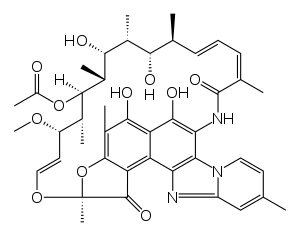
| Formula | C43H51N3O11 |
| Molar mass | 785.879 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Rifaximin, sold under the trade name Xifaxan among others, is an antibiotic used to treat traveler's diarrhea, irritable bowel syndrome, and hepatic encephalopathy.[1] It has poor absorption when taken by mouth.
It is based on rifamycin. Rifaximin was approved for medical use in the United States in 2004.[1] In the United States it costs 62.13 USD per day for 1100 mg of rifaximin (1,864.00 USD per month) as of January 2017.[2] In Russia as of 2016 a similar dose costs 231.25 RUB (approximately 4 USD).[3]
Medical uses
Rifaximin may be used to treat and prevent traveler's diarrhea.[4][5]
Irritable bowel syndrome
Rifaximin possesses anti-inflammatory and antibacterial properties and additionally, it is a nonabsorable antibiotic that acts locally in the gut. These properties make it efficacious in relieving chronic functional symptoms of non-constipation type irritable bowel syndrome (IBS). It appears to retain its therapeutic properties for this indication, even after repeated courses.[6][7] Rifaximin is particularly indicated where small intestine bacterial overgrowth is suspected of involvement in a person’s IBS. Symptom relief or improvement can be obtained for abdominal pain, bloating, and stool consistency. A drawback is that repeated courses may be necessary for relapse of symptoms.[7]
C. difficile infection
Rifaximin may also be a useful addition to vancomycin when treating patients with relapsing C. difficile infection.[8][9] However the quality of evidence of these studies was judged to be low.[10] Although exposure to rifamycins in the past may increase risk for resistance, so rifaximin should be avoided in such cases.
Hepatic encephalopathy
In the United States, rifaximin has orphan drug status for the treatment of hepatic encephalopathy.[11] Although high-quality evidence is still lacking, rifaximin appears to be as effective as or more effective than other available treatments for hepatic encephalopathy (such as lactulose), is better tolerated, and may work faster.[12] Rifaximin is taken by mouth. It has minimal side effects, prevents reoccurring encephalopathy, and is associated with high patient satisfaction. Patients are more compliant and satisfied to take this medication than any other due to minimal side effects, prolonged remission, and overall cost.[13] The drawbacks to rifaximin are increased cost and lack of robust clinical trials for HE without combination lactulose therapy.
Side effects
Rifaximin has few side effects.[7] Side effects are generally mild and uncommon;[6] this is largely because very little of the drug is absorbed from the gut meaning systemic side effects are absent.[7] Clostridium difficile infection does not typically result from rifaximin therapy unless risk factors such as immunosuppression and hospitalisation are present. Rifaximin is active against Clostridium difficile.[6]
Interactions
Rifaximin is not significantly absorbed from the gut and therefore does not have significant interactions with other drugs in people with normal liver function.[6]
Mechanism of action
Rifaximin interferes with transcription by binding to the β-subunit of bacterial RNA polymerase.[14] This results in the blockage of the translocation step that normally follows the formation of the first phosphodiester bond, which occurs in the transcription process.[15]
Rifaximin has a very low bioavailability due to it is poorly absorbed after oral administration.[7] Because rifaximin is absorbed poorly, most of the drug taken orally stays in the gastrointestinal tract where the infection takes place.[16] Because of this local action within the gut and the lack of horizontal transfer of resistant genes the development of bacterial resistance is rare.[6]
Availability
Rifaximin is a low cost drug.[7] In the United States, Salix Pharmaceuticals holds a US Patent for rifaximin and markets the drug under the name Xifaxan.[17][18] In addition to receiving FDA approval for traveler’s diarrhea and (marketing approved for)[18] hepatic encephalopathy, rifaximin received FDA approval for IBS in May 2015.[19] No generic formulation is available in the US and none has appeared due to the fact that the FDA approval process was ongoing. If rifaximin receives full FDA approval for hepatic encephalopathy it is likely that Salix will maintain marketing exclusivity and be protected from generic formulations until March 24, 2017.[18]
Rifaximin is approved in 33 countries for GI disorders.[20][21] On August 13, 2013, Health Canada issued a Notice of Compliance to Salix Pharmaceuticals Inc. for the drug product Zaxine.[22] In India it is available under the brand names Ciboz and Xifapill. In Russia and Ukraine the drug is sold under the name Alfa Normix (Альфа Нормикс), produced by Alfa Wassermann S.p.A (Italy).[23]
References
- 1 2 "Rifaximin". The American Society of Health-System Pharmacists. Retrieved 8 January 2017.
- ↑ "NADAC as of 2017-01-25". Centers for Medicare and Medicaid Services. Retrieved 25 January 2017.
- ↑ "Drug prices archive of the administration of the city of Krasnodar" (in Russian).
- ↑ "Xifaxan label information" (PDF). Retrieved 15 November 2008.
- ↑ DuPont HL (July 2007). "Therapy for and prevention of traveler's diarrhea". Clinical Infectious Diseases. 45 (Suppl 1): S78–84. doi:10.1086/518155. PMID 17582576.
- 1 2 3 4 5 Ponziani FR, Pecere S, Lopetuso L, Scaldaferri F, Cammarota G, Gasbarrini A (July 2016). "Rifaximin for the treatment of irritable bowel syndrome - a drug safety evaluation". Expert Opinion on Drug Safety. 15 (7): 983–91. doi:10.1080/14740338.2016.1186639. PMID 27149541.
- 1 2 3 4 5 6 Song KH, Jung HK, Kim HJ, Koo HS, Kwon YH, Shin HD, et al. (April 2018). "Clinical Practice Guidelines for Irritable Bowel Syndrome in Korea, 2017 Revised Edition". Journal of Neurogastroenterology and Motility. 24 (2): 197–215. doi:10.5056/jnm17145. PMC 5885719. PMID 29605976.
- ↑ Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN (March 2007). "Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin". Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 44 (6): 846–8. doi:10.1086/511870. PMID 17304459.
- ↑ Garey KW, Ghantoji SS, Shah DN, Habib M, Arora V, Jiang ZD, DuPont HL (December 2011). "A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection". The Journal of Antimicrobial Chemotherapy. 66 (12): 2850–5. doi:10.1093/jac/dkr377. PMID 21948965.
- ↑ Nelson RL, Suda KJ, Evans CT (March 2017). "Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults". The Cochrane Database of Systematic Reviews. 3: CD004610. doi:10.1002/14651858.CD004610.pub5. PMID 28257555.
- ↑ Wolf DC (2007-01-09). "Hepatic Encephalopathy". eMedicine. WebMD. Retrieved 2007-02-15.
- ↑ Lawrence KR, Klee JA (August 2008). "Rifaximin for the treatment of hepatic encephalopathy". Pharmacotherapy. 28 (8): 1019–32. doi:10.1592/phco.28.8.1019. PMID 18657018. Free full text with registration at Medscape.
- ↑ Kimer N, Krag A, Gluud LL (March 2014). "Safety, efficacy, and patient acceptability of rifaximin for hepatic encephalopathy". Patient Preference and Adherence. 8: 331–8. doi:10.2147/PPA.S41565. PMC 3964161. PMID 24672227.
- ↑ PharmD, Benjamin Barner,; PharmD, Brett Feret, (1 July 2010). "Rifaximin: A nonabsorbable, broad-spectrum antibiotic for reduction in the risk for recurrence of overt hepatic encephalopathy".
- ↑ "Rifaximin". DrugBank. 22 March 2017.
- ↑ Taylor DN (December 2005). "Poorly absorbed antibiotics for the treatment of traveler's diarrhea". Clinical Infectious Diseases. 41 Suppl 8 (Supplement_8): S564–70. doi:10.1086/432953. PMID 16267720.
- ↑ "XIFAXAN (Rifaximin) 550 mg - Reduce Overt Hepatic Encephalopathy Recurrences". Salix Pharmaceuticals.
- 1 2 3 "Product Details for NDA 022554". Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration.
- ↑ "Press Announcements - FDA approves two therapies to treat IBS-D". U.S. Food and Drug Administration.
- ↑ "Xifaxan® (rifaximin) Tablets 550 mg for Hepatic Encephalopathy" (PDF). United States Food and Drug Administration Gastrointestinal Drugs Advisory Committee. 23 February 2010. Archived from the original (PDF) on 14 January 2017.
- ↑ "Pharmaceutical News & Media - Salix Pharmaceuticals".
- ↑ "Summary Basis of Decision (SBD): Zaxine". Government of Canada, Health Canada, Health Products and Food Branch, Therapeutic Products Directorate, Bureau of Gastroenterology Infection and Viral Diseases. 2013.
- ↑ "Alfa Normix". Russian medical server.