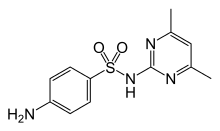
Sulfadimidine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard |
100.000.315 |
| Chemical and physical data | |
| Formula | C12H14N4O2S |
| Molar mass | 278.33 g/mol |
| 3D model (JSmol) | |
| Melting point | 176 °C (349 °F) |
| |
| |
| (verify) | |
Sulfadimidine or sulfamethazine is a sulfonamide antibacterial.
There are non-standardizeda abbreviations for it as "sulfadimidine" (abbreviated SDI[1][2] and more commonly but less reliablyb SDD[3][4]) and as "sulfamethazine" (abbreviated SMT[5][6] and more commonly but less reliablyc SMZ[7][8]). Other names include sulfadimerazine, sulfadimezine, and sulphadimethylpyrimidine.
Availability
In Pakistan, it is available with the brand name of "Sanadine-33.3 %" as potential treatment of E-coli enteritis in veterinary medicine. [9]
Notes
- ^a Abbreviations are not found in the databases (such as ChemDB, ChemIDplus, PubChem), but often seen in the published literature.
- ^b "SDD" is not found in databases, but often seen in the published literature; it could however be confused with Tiferron/Sodium catechol sulfate (1,2-Dihydroxybenzene-3,5-disulfonic acid disodium Salt), uncommon but found officially abbreviated SDD in the ChemIDplus database.[9]
- ^c "SMZ" is not found in databases, but often seen in the published literature; it could however be confused with sulfamethoxazole, also seen abbreviated SMZ.
- ^[9] http://www.sannalabs.com/productslist.htm
References
- ↑ Romváry, A; Simon, F (1992). "Sulfonamide residues in eggs". Acta veterinaria Hungarica. 40 (1–2): 99–106. ISSN 0236-6290. PMID 1476095.
- ↑ Reddy; Jain, S. K.; Uppal, R. P. (1988). "Pharmacokinetic studies of sulphonamides in poultry". Indian Journal of Animal Sciences.
- ↑ Kamakura, K; Hasegawa, M; Koiguchi, S; Miyata, M; Okamoto, K; Narita, M; Hirahara, Y; Yamana, T; et al. (1993). "Studies on the identification of sulfadimidine in pork by high performance liquid chromatography with photodiode array detector and gas chromatograph-mass spectrometry". Eisei Shikenjo hokoku. Bulletin of National Institute of Hygienic Sciences (111): 61–5. ISSN 0077-4715. PMID 7920569.
- ↑ Garg, SK; Ghosh, SS; Mathur, VS (Jan 1986). "Comparative pharmacokinetic study of four different sulfonamides in combination with trimethoprim in human volunteers". International journal of clinical pharmacology, therapy, and toxicology. 24 (1): 23–5. ISSN 0174-4879. PMID 3485584.
- ↑ Peña, MS; Salinas, F; Mahedero, MC; Aaron, JJ (Feb 1994). "Solvent effect on the determination of sulfamethazine by room-temperature photochemically induced fluorescence". Talanta. 41 (2): 233–6. doi:10.1016/0039-9140(94)80113-4. ISSN 0039-9140. PMID 18965913.
- ↑ Kaniou, S; Pitarakis, K; Barlagianni, I; Poulios, I (Jul 2005). "Photocatalytic oxidation of sulfamethazine". Chemosphere. 60 (3): 372–80. doi:10.1016/j.chemosphere.2004.11.069. ISSN 0045-6535. PMID 15924956.
- ↑ Calvo, R; Sarabia, S; Carlos, R; Du Souich, P (Mar 1987). "Sulfamethazine absorption and disposition: effect of surgical procedures for gastroduodenal ulcers". Biopharmaceutics & drug disposition. 8 (2): 115–24. doi:10.1002/bdd.2510080203. ISSN 0142-2782. PMID 3593892.
- ↑ De Liguoro, M; Fioretto, B; Poltronieri, C; Gallina, G (Jun 2009). "The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim". Chemosphere. 75 (11): 1519–24. doi:10.1016/j.chemosphere.2009.02.002. ISSN 0045-6535. PMID 19269673.
- ↑ http://chem.sis.nlm.nih.gov/chemidplus/ProxyServlet?objectHandle=Search&actionHandle=getAll3DMViewFiles&nextPage=jsp%2Fcommon%2FChemFull.jsp%3FcalledFrom%3Dlite&chemid=000149451&formatType=_3D
Further reading
- ChemDB. "Sulfamethazine", ChemDB, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH)
- Sulfadimidine in the ChemIDplus database
- PubChem. "Sulfamethazine - Substance Summary", PubChem, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), National Institutes of Health (NIH)
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.