Ornidazole
 | |
| Clinical data | |
|---|---|
| Trade names | Xynor |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Metabolized via the liver, excreted in the Urine and Feces |
| Excretion | 85% of single oral dose is eliminated with 5 days - Urine (63%) and Feces (22%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.037.099 |
| Chemical and physical data | |
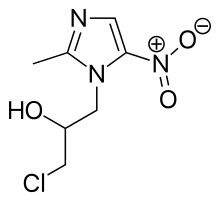
| Formula | C7H10ClN3O3 |
| Molar mass | 219.63 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ornidazole is an antibiotic used to treat some protozoan infections. It has also been investigated for use in Crohn's disease after bowel resection.[1]
Synthesis is a straightforward reaction between 2-methyl-nitroimidazole and epichlorohydrin under acid catalyst conditions.[2]
Mechanism of action and susceptible organisms
After passive absorption into bacterium cell, the nitro group of ornidazole is reduced to an amine group by ferrodoxin-type redox systems. The formation of redox intermediate intracellular metabolites is believed to be the key component responsible for killing microorganisms. The drug is active against anaerobic bacteria including Peptostreptococcus, Clostridium, Bacteroides fragilis, Prevotella, Porphyromonas gingivalis, and Fusobacterium as well as protozoa including Entamoeba histolytica, Trichomonas vaginalis, and Giardia lamblia.[3]
References
- ↑ Rutgeerts P, Van Assche G, Vermeire S, et al. (April 2005). "Ornidazole for prophylaxis of postoperative Crohn's disease recurrence: a randomized, double-blind, placebo-controlled trial". Gastroenterology. 128 (4): 856–61. doi:10.1053/j.gastro.2005.01.010. PMID 15825069.
- ↑ Hoffer, Max; Grunberg, Emanuel (1974). "Synthesis and antiprotozoal activity of 1-(3-chloro-2-hydroxypropyl)-substituted nitroimidazoles". Journal of Medicinal Chemistry. 17 (9): 1019–1020. doi:10.1021/jm00255a026. ISSN 0022-2623.
- ↑ http://webcache.googleusercontent.com/search?q=cache:OFkC_pjHLZ4J:www.panacea-biotec.com/product-pdf/Ocimix_28-11-2010.pdf