Oprozomib
 | |
| Clinical data | |
|---|---|
| Pronunciation | /oʊˈprɒzoʊmɪb/ oh-PROZ-oh-mib |
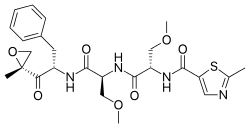
| Synonyms | O-methyl-N-(2-methyl-1,3-thiazol-5-carbonyl)-L-seryl-O-methyl-N-{(2S)-1-[(2R)-2-methyloxiran-2-yl]-1-oxo-3-phenylpropan-2-yl}-L-serinamide |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C25H32N4O7S |
| Molar mass | 532.61 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Oprozomib[1] (codenamed ONX 0912 and PR-047) is an orally active second-generation proteasome inhibitor developed by Proteolix, which was acquired by Onyx Pharmaceuticals, an Amgen subsidiary, in 2009. It selectively inhibits chymotrypsin-like activity of both the constitutive proteasome (PSMB5) and immunoproteasome (LMP7).[2]
It is being investigated for the treatment of hematologic malignancies, specifically, multiple myeloma, with Phase 1b studies ongoing (as of February 16, 2016).[3] Being an epoxyketone derivative, oprozomib is structurally related to carfilzomib and has the added benefit of being orally bioavailable. Like carfilzomib, it is active against bortezomib-resistant multiple myeloma cells.[4]
Oprozomib was granted orphan drug status for the treatment of Waldenstrom's macroglobulinaemia and multiple myeloma in 2014.[5]
See also
- Ixazomib (trade name Ninlaro) — an orally available boronic acid-derived proteasome inhibitor approved for the treatment of multiple myeloma
References
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed International Nonproprietary Names: List 107" (PDF). World Health Organization. p. 193. Retrieved 24 April 2016.
- ↑ Zhou, Han-Jie; Aujay, Monette A.; Bennett, Mark K.; Dajee, Maya; Demo, Susan D.; Fang, Ying; Ho, Mark N.; Jiang, Jing; Kirk, Christopher J.; Laidig, Guy J.; Lewis, Evan R.; Lu, Yan; Muchamuel, Tony; Parlati, Francesco; Ring, Eileen; Shenk, Kevin D.; Shields, Jamie; Shwonek, Peter J.; Stanton, Timothy; Sun, Congcong M.; Sylvain, Catherine; Woo, Tina M.; Yang, Jinfu (14 May 2009). "Design and Synthesis of an Orally Bioavailable and Selective Peptide Epoxyketone Proteasome Inhibitor (PR-047)". Journal of Medicinal Chemistry. 52 (9): 3028–38. doi:10.1021/jm801329v. PMID 19348473.
- ↑ "Amgen Pipeline Chart". Amgen Inc. February 16, 2016. p. 3. Retrieved 24 April 2016.
- ↑ Chauhan, D; Singh, AV; Aujay, M; Kirk, CJ; Bandi, M; Ciccarelli, B; Raje, N; Richardson, P; Anderson, KC (30 August 2010). "A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma". Blood. 116 (23): 4906–15. doi:10.1182/blood-2010-04-276626. PMC 3321748. PMID 20805366.
- ↑ http://webcache.googleusercontent.com/search?q=cache:StNuTZ7KAPQJ:adisinsight.springer.com/drugs/800032242+&cd=24&hl=en&ct=clnk&gl=us