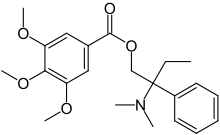
Trimebutine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard |
100.049.354 |
| Chemical and physical data | |
| Formula | C22H29NO5 |
| Molar mass | 387.47 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Trimebutine is a drug with antimuscarinic and weak mu opioid agonist effects.[1] The maleic acid salt of trimebutine is marketed under the trademarks of Debridat, Recutin, Polybutin,[2] or Modulon, for the treatment of irritable bowel syndrome and other gastrointestinal disorders.
The major product from drug metabolism of trimebutine in human beings is nortrimebutine,[3] which comes from removal of one of the methyl groups attached to the nitrogen atom.
Trimebutine exerts its effects in part due to causing a premature activation of phase III of the migrating motor complex in the digestive tract.[4]
Both trimebutine and its metabolite are commercially available.
See also
References
- ↑ Kaneto H, Takahashi M, Watanabe J. The opioid receptor selectivity for trimebutine in isolated tissues experiments and receptor binding studies. Journal of Pharmacobiodynamics. 1990 Jul;13(7):448-53. PMID 1963196
- ↑ Ok Hwa Jhee, Yun Sik Lee, Leslie M. Shaw, Yong Cheol Jeon, Min Ho Lee, Seung Hoon Lee and Ju Seop Kang The Pharmacokinetic and bioequivalence evaluation of two formulations of 100 mg trimebutine maleate (Recutin and Polybutin) in healthy male volunteers using the LC–MS/MS method. Clinica Chimica Acta. 2007 Jan;375(1-2):69-75. PMID 16854404
- ↑ F.J. Roman, S. Lanet, J. Hamon, G. Brunelle, A. Maurin, P. Champeroux, S. Richard, N. Alessandri, and M. Gola Pharmacological Properties of Trimebutine and N-Monodesmethyltrimebutine. The Journal of Pharmacology and Experimental Therapeutics 1999;289:1391–1397. PMID 10336531
- ↑ Hiyama, T.; Yoshihara, M.; Tanaka, S.; Haruma, K.; Chayama, K. (Apr 2009). "Effectiveness of prokinetic agents against diseases external to the gastrointestinal tract" (PDF). J Gastroenterol Hepatol. 24 (4): 537–46. doi:10.1111/j.1440-1746.2009.05780.x. PMID 19220673.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.