Mineral (nutrient)
In the context of nutrition, a mineral is a chemical element required as an essential nutrient by organisms to perform functions necessary for life.[1][2] Minerals originate in the earth and cannot be made by living organisms.[3] Plants get minerals from soil.[3] Most of the minerals in a human diet come from eating plants and animals or from drinking water.[3] As a group, minerals are one of the four groups of essential nutrients, the others of which are vitamins, essential fatty acids, and essential amino acids.[4] The five major minerals in the human body are calcium, phosphorus, potassium, sodium, and magnesium.[1] All of the remaining elements in a human body are called "trace elements". The trace elements that have a specific biochemical function in the human body are sulfur, iron, chlorine, cobalt, copper, zinc, manganese, molybdenum, iodine and selenium.[5]
Most chemical elements that are ingested by organisms are in the form of simple compounds. Plants absorb dissolved elements in soils, which are subsequently ingested by the herbivores and omnivores that eat them, and the elements move up the food chain. Larger organisms may also consume soil (geophagia) or use mineral resources, such as salt licks, to obtain limited minerals unavailable through other dietary sources.
Bacteria and fungi play an essential role in the weathering of primary elements that results in the release of nutrients for their own nutrition and for the nutrition of other species in the ecological food chain. One element, cobalt, is available for use by animals only after having been processed into complex molecules (e.g., vitamin B12) by bacteria. Minerals are used by animals and microorganisms for the process of mineralizing structures, called "biomineralization", used to construct bones, seashells, eggshells, exoskeletons and mollusc shells.[6]
Essential chemical elements for humans
At least twenty chemical elements are known to be required to support human biochemical processes by serving structural and functional roles as well as electrolytes.[7] However, as many as twenty-nine elements in total (including hydrogen, carbon, nitrogen and oxygen) are suggested to be used by mammals, as inferred by biochemical and uptake studies.[8] Calcium makes up 920 to 1200 grams of adult body weight, with 99% of it contained in bones and teeth.[1] Phosphorus makes up about 1% of a person's body weight.[9] The other major minerals (potassium, sodium, chlorine, sulfur and magnesium) make up only about 0.85% of the weight of the body. Together these eleven chemical elements (H, C, N, O, Ca, P, K, Na, Cl, S, Mg) make up 99.85% of the body. There is not scientific consensus on whether chromium is an essential trace element. The United States and Japan designate chromium as an essential nutrient,[10][11] but the European Food Safety Authority (EFSA), representing the European Union, reviewed the question in 2014 and does not agree.[12]
Most of the known and suggested mineral nutrients are of relatively low atomic weight, and are reasonably common on land, or for sodium and iodine, in the ocean:
| H | He | |||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |
| Cs | Ba | La | * | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og |
| * | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ||||
| ** | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | ||||
Quantity elements |
Deemed essential trace element by U.S., not by European Union |
Suggested function from deprivation effects or active metabolic handling, but no clearly-identified biochemical function in humans |
Limited circumstantial evidence for trace benefits or biological action in mammals |
No evidence for biological action in mammals, but essential in some lower organisms. (In the case of lanthanum, the definition of an essential nutrient as being indispensable and irreplaceable is not completely applicable due to the extreme similarity of the lanthanides. Thus Ce, Pr, and Nd may be substituted for La without ill effects for organisms using La, and the smaller Sm, Eu, and Gd may also be similarly substituted but cause slower growth.) |
Roles in biological processes
| Dietary element | RDA (US) [mg][13] | UL (US and EU) [mg][14][15][16] | Category | High nutrient density dietary sources |
Term for deficiency | Term for excess |
|---|---|---|---|---|---|---|
| Potassium | 4700 | NE; NE | A systemic electrolyte and is essential in coregulating ATP with sodium | Sweet potato, tomato, potato, beans, lentils, dairy products, seafood, banana, prune, carrot, orange[17] | hypokalemia | hyperkalemia |
| Chlorine | 2300 | 3600; NE | Needed for production of hydrochloric acid in the stomach and in cellular pump functions | Table salt (sodium chloride) is the main dietary source. | hypochloremia | hyperchloremia |
| Sodium | 1500 | 2300; NE | A systemic electrolyte and is essential in coregulating ATP with potassium | Table salt (sodium chloride, the main source), sea vegetables, milk, and spinach. | hyponatremia | hypernatremia |
| Calcium | 1200 | 2500; 2500 | Needed for muscle, heart and digestive system health, builds bone, supports synthesis and function of blood cells | Dairy products, eggs, canned fish with bones (salmon, sardines), green leafy vegetables, nuts, seeds, tofu, thyme, oregano, dill, cinnamon.[18] | hypocalcaemia | hypercalcaemia |
| Phosphorus | 700 | 4000; 4000 | A component of bones (see apatite), cells, in energy processing, in DNA and ATP (as phosphate) and many other functions | Red meat, dairy foods, fish, poultry, bread, rice, oats.[19][20] In biological contexts, usually seen as phosphate[21] | hypophosphatemia | hyperphosphatemia |
| Magnesium | 420 | 350; 250 | Required for processing ATP and for bones | Spinach, legumes, nuts, seeds, whole grains, peanut butter, avocado[22] | hypomagnesemia, magnesium deficiency |
hypermagnesemia |
| Iron | 18 | 45; NE | Required for many proteins and enzymes, notably hemoglobin to prevent anemia | Meat, seafood, nuts, beans, dark chocolate[23] | iron deficiency | iron overload disorder |
| Zinc | 11 | 40; 25 | Pervasive and required for several enzymes such as carboxypeptidase, liver alcohol dehydrogenase, and carbonic anhydrase | Oysters*, red meat, poultry, nuts, whole grains, dairy products[24] | zinc deficiency | zinc toxicity |
| Manganese | 2.3 | 11; NE | A cofactor in enzyme functions | Grains, legumes, seeds, nuts, leafy vegetables, tea, coffee[25] | manganese deficiency | manganism |
| Copper | 0.9 | 10; 5 | Required component of many redox enzymes, including cytochrome c oxidase | Liver, seafood, oysters, nuts, seeds; some: whole grains, legumes[25] | copper deficiency | copper toxicity |
| Iodine | 0.150 | 1.1; 0.6 | Required for synthesis of thyroid hormones, thyroxine and triiodothyronine and to prevent goiter: | Seaweed (kelp or kombu)*, grains, eggs, iodized salt[26] | iodine deficiency | iodism Hyperthyroidism[27] |
| Chromium | 0.035 | NE; NE | Involved in glucose and lipid metabolism, although its mechanisms of action in the body and the amounts needed for optimal health are not well-defined[28][29] | Broccoli, grape juice (especially red), meat, whole grain products[30] | Chromium deficiency | Chromium toxicity |
| Molybdenum | 0.045 | 2; 0.6 | The oxidases xanthine oxidase, aldehyde oxidase, and sulfite oxidase[31] | Legumes, whole grains, nuts[25] | molybdenum deficiency | molybdenum toxicity[32] |
| Selenium | 0.055 | 0.4; 0.3 | Essential to activity of antioxidant enzymes like glutathione peroxidase | Brazil nuts, seafoods, organ meats, meats, grains, dairy products, eggs[33] | selenium deficiency | selenosis |
| Cobalt | none | NE; NE | Required in the synthesis of vitamin B12, but because bacteria are required to synthesize the vitamin, it is usually considered part of vitamin B12 which comes from eating animals and animal-sourced foods (eggs...) | Cobalt poisoning |
RDA = Recommended Dietary Allowance; UL = Tolerable upper intake level; Figures shown are for adults age 31-50, male or female neither pregnant nor lactating
* One serving of seaweed exceeds the US UL of 1100 μg but not the 3000 μg UL set by Japan.[34]
Blood concentrations of minerals
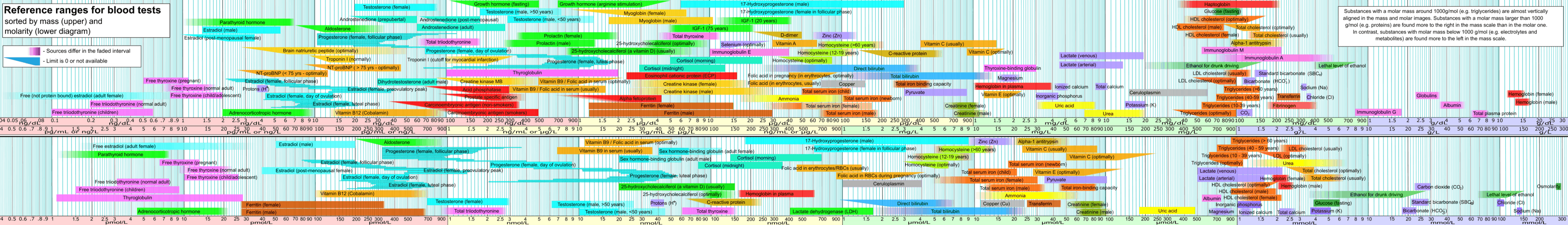
Minerals are present in a healthy human being's blood at certain mass and molar concentrations. The figure below presents the concentrations of each of the chemical elements discussed in this article, from center-right to the right. Depending on the concentrations, some are in upper part of the picture, while others are in the lower part. The figure includes the relative values of other constituents of blood such as hormones. In the figure, minerals are color highlighted in purple.
Dietary nutrition
Dietitians may recommend that minerals are best supplied by ingesting specific foods rich with the chemical element(s) of interest. The elements may be naturally present in the food (e.g., calcium in dairy milk) or added to the food (e.g., orange juice fortified with calcium; iodized salt fortified with iodine). Dietary supplements can be formulated to contain several different chemical elements (as compounds), a combination of vitamins and/or other chemical compounds, or a single element (as a compound or mixture of compounds), such as calcium (calcium carbonate, calcium citrate) or magnesium (magnesium oxide), or iron (ferrous sulfate, iron bis-glycinate).
The dietary focus on chemical elements derives from an interest in supporting the biochemical reactions of metabolism with the required elemental components.[35] Appropriate intake levels of certain chemical elements have been demonstrated to be required to maintain optimal health. Diet can meet all the body's chemical element requirements, although supplements can be used when some recommendations are not adequately met by the diet. An example would be a diet low in dairy products, and hence not meeting the recommendation for calcium.
Elements considered possibly essential but not confirmed
Many ultratrace elements have been suggested as essential, but such claims have usually not been confirmed. Definitive evidence for efficacy comes from the characterization of a biomolecule containing the element with an identifiable and testable function.[5] One problem with identifying efficacy is that some elements are innocuous at low concentrations and are pervasive (examples: silicon and nickel in solid and dust), so proof of efficacy is lacking because deficiencies are difficult to reproduce.[35] Ultratrace elements of some minerals such as silicon and boron are known to have a role but the exact biochemical nature is unknown, and others such as arsenic are suspected to have a role in health, but with weaker evidence.[5]
| Element | Description | Excess |
|---|---|---|
| Bromine | Possibly important to basement membrane architecture and tissue development, as a needed catalyst to make collagen IV.[36] | bromism |
| Arsenic | Essential in rat, hamster, goat and chicken models, but no biochemical mechanism known in humans.[37] | arsenic poisoning |
| Nickel | Nickel is an essential component of several enzymes, including urease and hydrogenase.[38] Although not required by humans, some are thought to be required by gut bacteria, such as urease required by some varieties of Bifidobacterium.[39] In humans, nickel may be a cofactor or structural component of certain metalloenzymes involved in hydrolysis, redox rections, and gene expression. Nickel deficiency depressed growth in goats, pigs, and sheep, and diminished circulating thyroid hormone concentration in rats.[40] | Nickel toxicity |
| Fluorine | Fluorine (as fluoride) is not considered an essential element because humans do not require it for growth or to sustain life. Research indicates that the primary dental benefit from fluoride occurs at the surface from topical exposure.[41][42] Of the minerals in this table, fluoride is the only one for which the U.S. Institute of Medicine has established an Adequate Intake.[43] | Fluoride poisoning |
| Boron | Boron is an essential plant nutrient, required primarily for maintaining the integrity of cell walls.[44][45][46] Boron has been shown to be essential to complete the life cycle in representatives of all phylogenetic kingdoms, including the model species Danio rerio (zebrafish) and Xenopus laevis (African clawed frog).[38][47] In animals, supplemental boron has been shown to reduce calcium excretion and activate vitamin D.[48] | Nontoxic |
| Lithium | It is not known whether lithium has a physiological role in any species,[49] but nutritional studies in mammals have indicated its importance to health, leading to a suggestion that it be classed as an essential trace element. | Lithium toxicity |
| Strontium | Strontium has been found to be involved in the utilization of calcium in the body. It has promoting action on calcium uptake into bone at moderate dietary strontium levels, but a rachitogenic (rickets-producing) action at higher dietary levels.[50] | Rachitogenic (causing Rickets) |
| Other | Silicon and vanadium have established, albeit specialized, biochemical roles as structural or functional cofactors in other organisms, and are possibly, even probably, used by mammals (including humans). By contrast, tungsten, lanthanum, and cadmium have specialized biochemical uses in certain lower organisms, but these elements appear not to be utilized by humans.[8] Other elements considered to be possibly essential include aluminium, germanium, lead, rubidium, and tin.[38][51][52] | Multiple |
Mineral ecology
Minerals can be bioengineered by bacteria which act on metals to catalyze mineral dissolution and precipitation.[53] Mineral nutrients are recycled by bacteria distributed throughout soils, oceans, freshwater, groundwater, and glacier meltwater systems worldwide.[53][54] Bacteria absorb dissolved organic matter containing minerals as they scavenge phytoplankton blooms.[54] Mineral nutrients cycle through this marine food chain, from bacteria and phytoplankton to flagellates and zooplankton, which are then eaten by other marine life.[53][54] In terrestrial ecosystems, fungi have similar roles as bacteria, mobilizing minerals from matter inaccessible by other organisms, then transporting the acquired nutrients to local ecosystems.[55][56]
See also
References
- 1 2 3 Berdanier, Carolyn D.; Dwyer, Johanna T.; Heber, David (2013). Handbook of Nutrition and Food (3rd ed.). CRC Press. p. 199. ISBN 978-1-4665-0572-8. Retrieved 3 July 2016.
- ↑ "Minerals". MedlinePlus, National Library of Medicine, US National Institutes of Health. 22 December 2016. Retrieved 24 December 2016.
- 1 2 3 "Minerals". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. 2016.
- ↑ "Vitamin and mineral supplement fact sheets". Office of Dietary Supplements, US National Institutes of Health, Bethesda, MD. 2016. Retrieved 19 December 2016.
- 1 2 3 Berdanier, Carolyn D.; Dwyer, Johanna T.; Heber, David (19 April 2016). Handbook of Nutrition and Food, Third Edition. CRC Press. pp. 211–224. ISBN 978-1-4665-0572-8. Retrieved 3 July 2016.
- ↑ Harris, Ph.D., Edward D. (1 January 2014). Minerals in Food Nutrition, Metabolism, Bioactivity (chapter 3.4) (1st ed.). Lancaster, PA: DEStech Publications, Inc. p. 378. ISBN 978-1-932078-97-8. Retrieved 27 December 2016.
- ↑ Nelson, David L.; Michael M. Cox (2000-02-15). Lehninger Principles of Biochemistry, Third Edition (3 Har/Com ed.). W. H. Freeman. p. 1200. ISBN 1-57259-931-6.
- 1 2 Ultratrace minerals. Authors: Nielsen, Forrest H. USDA, ARS Source: Modern nutrition in health and disease / editors, Maurice E. Shils ... et al.. Baltimore : Williams & Wilkins, c1999., p. 283-303. Issue Date: 1999 URI:
- ↑ "Phosphorus in diet". MedlinePlus, National Library of Medicine, US National Institutes of Health. 2 December 2016. Retrieved 24 December 2016.
- ↑ Chromium. IN: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Chromium, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Chromium. Institute of Medicine (US) Panel on Micronutrients. National Academy Press. 2001, PP.197-223.
- ↑ Overview of Dietary Reference Intakes for Japanese (2015)
- ↑ "Scientific Opinion on Dietary Reference Values for chromium". European Food Safety Authority. September 18, 2014. Retrieved March 20, 2018.
- ↑ U.S. Food and Drug Administration 14. Appendix F(mg)
- ↑ Dietary Reference Intakes (DRIs): Elements Food and Nutrition Board, Institute of Medicine, National Academies (2011)(mg)
- ↑ Dietary Reference Intakes : Electrolytes and Water The National Academies (2004)
- ↑ Tolerable Upper Intake Levels For Vitamins And Minerals (PDF), European Food Safety Authority, 2006
- ↑ "Dietary Guidelines for Americans 2005: Appendix B-1. Food Sources of Potassium". United States Department of Agriculture. 2005.
- ↑ Adam Drewnowski (2010). "The Nutrient Rich Foods Index helps to identify healthy, affordable foods" (PDF). The American Journal of Clinical Nutrition. 91(suppl): 1095S–1101S.
- ↑ "NHS Choices:Vitamins and minerals – Others". Retrieved November 8, 2011.
- ↑ Corbridge, DE (1995-02-01). Phosphorus: An Outline of Its Chemistry, Biochemistry, and Technology (5th ed.). Amsterdam: Elsevier Science Pub Co. p. 1220. ISBN 0-444-89307-5.
- ↑ "Phosphorus". Linus Pauling Institute, Oregon State University. 2014. Retrieved 2018-09-08.
- ↑ "Magnesium—Fact Sheet for Health Professionals". National Institutes of Health. 2016.
- ↑ "Iron—Dietary Supplement Fact Sheet". National Institutes of Health. 2016.
- ↑ "Zinc—Fact Sheet for Health Professionals". National Institutes of Health. 2016.
- 1 2 3 Schlenker, Eleanor; Gilbert, Joyce Ann (28 August 2014). Williams' Essentials of Nutrition and Diet Therapy. Elsevier Health Sciences. pp. 162–3. ISBN 978-0-323-29401-0. Retrieved 15 July 2016.
- ↑ "Iodine—Fact Sheet for Health Professionals". National Institutes of Health. 2016.
- ↑ Jameson, J. Larry; De Groot, Leslie J. (25 February 2015). Endocrinology: Adult and Pediatric. Elsevier Health Sciences. p. 1510. ISBN 978-0-323-32195-2. Retrieved 14 July 2016.
- ↑ Kim, Myoung Jin; Anderson, John; Mallory, Caroline (1 February 2014). Human Nutrition. Jones & Bartlett Publishers. p. 241. ISBN 978-1-4496-4742-1. Retrieved 10 July 2016.
- ↑ Gropper, Sareen S.; Smith, Jack L. (1 June 2012). Advanced Nutrition and Human Metabolism. Cengage Learning. pp. 527–8. ISBN 1-133-10405-3. Retrieved 10 July 2016.
- ↑ "Chromium". Office of Dietary Supplements, US National Institutes of Health. 2016. Retrieved 10 July 2016.
- ↑ Sardesai VM (December 1993). "Molybdenum: an essential trace element". Nutr Clin Pract. 8 (6): 277–81. doi:10.1177/0115426593008006277. PMID 8302261.
- ↑ Momcilović, B. (September 1999). "A case report of acute human molybdenum toxicity from a dietary molybdenum supplement—a new member of the "Lucor metallicum" family". Archives of Industrial Hygiene and Toxicology. De Gruyter. 50 (3): 289–97. PMID 10649845.
- ↑ "Selenium—Fact Sheet for Health Professionals". National Institutes of Health. 2016.
- ↑ Overview of Dietary Reference Intakes for Japanese (2015) Minister of Health, Labour and Welfare, Japan| url = http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf
- 1 2 Lippard, SJ; Berg JM (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books. p. 411. ISBN 0-935702-72-5.
- ↑ A. Scott McCall; Christopher F. Cummings; Gautam Bhave; Roberto Vanacore; Andrea Page-McCaw; Billy G. Hudson (5 June 2014). "Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture". Cell. 157 (6): 1380–1392. doi:10.1016/j.cell.2014.05.009. PMC 4144415. PMID 24906154.
- ↑ Anke M. Arsenic. In: Mertz W. ed., Trace elements in human and Animal Nutrition, 5th ed. Orlando, FL: Academic Press, 1986, 347–372; Uthus E.O., Evidency for arsenical essentiality, Environ. Geochem. Health, 1992, 14:54–56; Uthus E.O., Arsenic essentiality and factors affecting its importance. In: Chappell W.R, Abernathy C.O, Cothern C.R. eds., Arsenic Exposure and Health. Northwood, UK: Science and Technology Letters, 1994, 199–208.
- 1 2 3 Berdanier, Carolyn D.; Dwyer, Johanna T.; Heber, David (19 April 2016). Handbook of Nutrition and Food, Third Edition. CRC Press. pp. 211–26. ISBN 978-1-4665-0572-8. Retrieved 3 July 2016.
- ↑ Sigel, Astrid; Sigel, Helmut; Sigel, Roland K. O. (27 January 2014). Interrelations between Essential Metal Ions and Human Diseases. Springer Science & Business Media. p. 349. ISBN 978-94-007-7500-8. Retrieved 4 July 2016.
- ↑ Institute of Medicine (29 September 2006). Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academies Press. pp. 313–19, 415–22. ISBN 978-0-309-15742-1. Retrieved 21 June 2016.
- ↑ Mitsuo Kakei, Toshiro Sakae and Masayoshi Yoshikawa (2012). "Aspects Regarding Fluoride Treatment for Reinforcement and Remineralization of Apatite Crystals". Journal of Hard Tissue Biology. 21 (3): 475–6. Retrieved 2017-06-01.
- ↑ Peter Loskill, Christian Zeitz, Samuel Grandthyll, Nicolas Thewes, Frank Müller, Markus Bischoff, Mathias, Herrmann, Karin Jacobs (2013). "Reduced Adhesion of Oral Bacteria on Hydroxyapatite by Fluoride Treatment". Langmuir. doi:10.1021/la4008558. Retrieved 2017-06-01.
- ↑ Institute of Medicine (1997). "Fluoride". Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. Washington, DC: The National Academies Press. pp. 288–313.
- ↑ Mahler, R. L. "Essential Plant Micronutrients. Boron in Idaho" (PDF). University of Idaho. Archived from the original (PDF) on 1 October 2009. Retrieved 2009-05-05.
- ↑ "Functions of Boron in Plant Nutrition" (PDF). U.S. Borax Inc. Archived from the original (PDF) on 20 March 2009.
- ↑ Blevins, Dale G.; Lukaszewski, KM (1998). "Functions of Boron in Plant Nutrition". Annual Review of Plant Physiology and Plant Molecular Biology. 49 (1): 481–500. doi:10.1146/annurev.arplant.49.1.481. PMID 15012243.
- ↑ Erdman, John W., Jr.; MacDonald, Ian A.; Zeisel, Steven H. (30 May 2012). Present Knowledge in Nutrition. John Wiley & Sons. p. 1324. ISBN 978-0-470-96310-4. Retrieved 4 July 2016.
- ↑ Nielsen, Forrest H. (1997). "Boron in human and animal nutrition". Plant and Soil. 193 (2): 199–208. doi:10.1023/A:1004276311956. ISSN 0032-079X.
- ↑ "Some Facts about Lithium". ENC Labs. Retrieved 2010-10-15.
- ↑ "The biological role of strontium". Retrieved 2010-10-06.
- ↑ Gottschlich, Michele M. (2001). The Science and Practice of Nutrition Support: A Case-based Core Curriculum. Kendall Hunt. p. 98. ISBN 978-0-7872-7680-5. Retrieved 9 July 2016.
- ↑ Insel, Paul M.; Turner, R. Elaine; Ross, Don (2004). Nutrition. Jones & Bartlett Learning. p. 499. ISBN 978-0-7637-0765-1. Retrieved 10 July 2016.
- 1 2 3 Warren, L. A.; Kauffman, M. E. (2003). "Microbial geoengineers". Science. 299 (5609): 1027–9. doi:10.1126/science.1072076. JSTOR 3833546. PMID 12586932.
- 1 2 3 Azam, F.; Fenchel, T.; Field, J. G.; Gray, J. S.; Meyer-Reil, L. A.; Thingstad, F. (1983). "The ecological role of water-column microbes in the sea" (PDF). Mar. Ecol. Prog. Ser. 10: 257–263. Bibcode:1983MEPS...10..257A. doi:10.3354/meps010257.
- ↑ J. Dighton (2007). "Nutrient Cycling by Saprotrophic Fungi in Terrestrial Habitats". In Kubicek, Christian P.; Druzhinina, Irina S. Environmental and microbial relationships (2nd ed.). Berlin: Springer. pp. 287–300. ISBN 978-3-540-71840-6.
- ↑ Gadd, G. M (2017). "The Geomycology of Elemental Cycling and Transformations in the Environment" (PDF). Microbiology Spectrum. 5 (1). doi:10.1128/microbiolspec.FUNK-0010-2016. PMID 28128071.
Further reading
- Humphry Bowen (1966) Trace Elements in Biochemistry. Academic Press.
- Humphrey Bowen (1979) Environmental Chemistry of the Elements. Academic Press, ISBN 0-12-120450-2.
External links
| Wikimedia Commons has media related to Dietary minerals. |