Iron–sulfur cluster
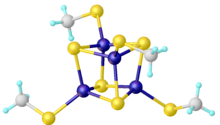
Iron–sulfur clusters are molecular ensembles of iron and sulfide. They are most often discussed in the context of the biological role for iron-sulfur proteins, which are pervasive.[2] Many Fe-S clusters are known in the area of organometallic chemistry and as precursors to synthetic analogues of the biological clusters (see Figure).
Biomimetic clusters
The most well characterized clusters have the formula [Fe4S4(SR)4]2−, which are known for many R substituents, and with many cations. Many variations have been prepared including the incomplete cubanes [Fe3S4(SR)3]3−.[3]
Organometallic clusters
Organometallic Fe-S clusters include the sulfido carbonyls with the formula Fe2S2(CO)6, H2Fe3S(CO)9, and Fe3S2(CO)9. Compounds are also known that incorporate cyclopentadienyl ligands, such as (C5H5)4Fe4S4.[4]

See also
References
- ↑ Axel Kern, Christian Näther, Felix Studt, Felix Tuczek (2004). "Application of a Universal Force Field to Mixed Fe/Mo−S/Se Cubane and Heterocubane Clusters. 1. Substitution of Sulfur by Selenium in the Series [Fe4X4(YCH3)4]2-; X = S/Se and Y = S/Se". Inorg. Chem. 43: 5003–5010. doi:10.1021/ic030347d.
- ↑ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ↑ Rao, P. V.; Holm, R. H. (2004). "Synthetic Analogues of the Active Sites of Iron-Sulfur Proteins". Chem. Rev. 104: 527─559. doi:10.1021/Cr020615+.
- ↑ Ogino, H., Inomata, S., Tobita, H. (1998). "Abiological Iron-Sulfur Clusters". Chem. Rev. 98: 2093. doi:10.1021/cr940081f.