Peroxymonosulfuric acid
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Peroxysulfuric acid Sulfuroperoxoic acid[1] | |
| Systematic IUPAC name | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.879 |
| EC Number | 231-766-6 |
| 101039 | |
PubChem CID |
|
| UN number | 1483 |
| |
| |
| Properties | |
| H 2SO 5 | |
| Molar mass | 114.078 g mol−1 |
| Appearance | White crystals |
| Density | 2.239 g cm−3 |
| Conjugate base | Peroxomonosulfate |
| Structure | |
| Tetrahedral at S | |
| Hazards | |
| Main hazards | strong oxidizer |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
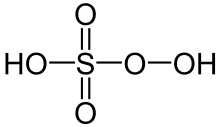
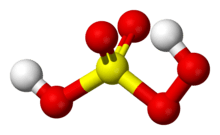
Peroxymonosulfuric acid, (H2SO5), also known as persulfuric acid, peroxysulfuric acid, or Caro's acid, is a liquid at room temperature. In this acid, the S(VI) center adopts its characteristic tetrahedral geometry; the connectivity is indicated by the formula HO–O–S(O)2–OH. It is one of the strongest oxidants known (E0 = +2.51 V) and is highly explosive.
H2SO5 is sometimes confused with H2S2O8, known as peroxydisulfuric acid. The disulfuric acid, which appears to be more widely used as its alkali metal salts, has the structure HO–S(O)2–O–O–S(O)2–OH.
History
H2SO5 was first described in 1898 by Heinrich Caro, after whom it is named.[3]
Synthesis and production
The laboratory scale preparation of Caro's acid involves the combination of Chlorosulfuric acid and hydrogen peroxide.
- H2O2 + ClSO2OH ⇌ H2SO5 + HCl [4]
Published patents include more than one reaction for preparation of Caro's acid, usually as an intermediate for the production of potassium monopersulfate (PMPS), a bleaching and oxidizing agent. One patent for production of Caro's acid for this purpose gives the following reaction:
- H2O2 + H2SO4 ⇌ H2SO5 + H2O [5]
Uses in industry
H2SO5 has been used for a variety of disinfectant and cleaning applications, e.g., swimming pool treatment and denture cleaning. Alkali metal salts of H2SO5 show promise for the delignification of wood.[6]
Ammonium, sodium, and potassium salts of H2SO5 are used in the plastic industry as polymerization initiators, etchants, desizing agents, soil conditioner, and for decolorizing and deodorizing oils.
Potassium peroxymonosulfate, KHSO5, is the potassium acid salt of peroxymonosulfuric acid. It is widely used as an oxidizing agent.
Dangers
Pure Caro's acid is highly explosive. Explosions have been reported at Brown University[7] and Sun Oil. As with all strong oxidizing agents, peroxysulfuric acid should be kept away from organic compounds such as ethers and ketones because of its ability to peroxidize the compound, creating a highly unstable molecule such as acetone peroxide.
See also
References
- 1 2 3 International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. p. 139. Electronic version.
- ↑ "peroxysulfuric acid (CHEBI:29286)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 20 November 2007. Retrieved 17 November 2011.
- ↑ Caro, H. (1898). "Zur Kenntniss der Oxydation aromatischer Amine" [[Contribution] to [our] knowledge of the oxidation of aromatic amines]. Zeitschrift für angewandte Chemie. 11 (36): 845–846. doi:10.1002/ange.18980113602.
- ↑ "Synthesis of Caro's acid". PrepChem.com. 2017-02-13. Retrieved 2018-10-12.
- ↑ A method and apparatus for producing a peroxyacid solution, retrieved 2018-10-12
- ↑ Springer, E. L.; McSweeny, J. D. (1993). "Treatment of softwood kraft pulps with peroxymonosulfate before oxygen delignification". Tappi Journal. 76 (8): 194–199. ISSN 0734-1415.
- ↑ J.O. Edwards (1955). "SAFETY". Chem. Eng. News. 33 (32): 3336. doi:10.1021/cen-v033n032.p3336.