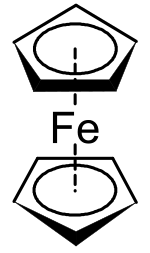
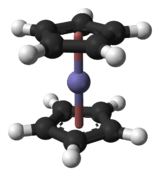
Ferrocene
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
ferrocene, bis(η5-cyclopentadienyl)iron | |||
| Other names
dicyclopentadienyl iron | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.764 | ||
PubChem CID |
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C10H10Fe | |||
| Molar mass | 186.04 g/mol | ||
| Appearance | light orange powder | ||
| Odor | camphor-like | ||
| Density | 1.107 g/cm3 (0 °C), 1.490 g/cm3 (20 °C)[1] | ||
| Melting point | 172.5 °C (342.5 °F; 445.6 K)[2] | ||
| Boiling point | 249 °C (480 °F; 522 K) | ||
| Insoluble in water, soluble in most organic solvents | |||
| log P | 2.04050 [3] | ||
| Hazards | |||
| Main hazards | Very hazardous in case of ingestion. Hazardous in case of skin contact (irritant), of eye contact (irritant), of inhalation[4] | ||
EU classification (DSD) (outdated) |
  | ||
| NFPA 704 | |||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) |
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[6] | ||
REL (Recommended) |
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[6] | ||
IDLH (Immediate danger) |
N.D.[6] | ||
| Related compounds | |||
Related compounds |
cobaltocene, nickelocene, chromocene, ruthenocene, osmocene, plumbocene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Ferrocene is an organometallic compound with the formula Fe(C5H5)2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds.[7][8] The rapid growth of organometallic chemistry is often attributed to the excitement arising from the discovery of ferrocene and its many analogues.
History
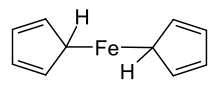
Ferrocene was first prepared unintentionally. In 1951, Peter Pauson and T. J. Kealy at Duquesne University reported the reaction of cyclopentadienyl magnesium bromide and ferric chloride with the goal of oxidatively coupling the diene to prepare fulvalene. Instead, they obtained a light orange powder of "remarkable stability".[9] A second group at British Oxygen also unknowingly discovered ferrocene. Miller, Tebboth and Tremaine were trying to synthesise amines from hydrocarbons such as cyclopentadiene and ammonia in a modification of the Haber process. They published this result in 1952 although the actual work was done three years earlier.[10][11][12] The stability of the new organoiron compound was accorded to the aromatic character of the negatively charged cyclopentadienyls, but they were not the ones to recognize the η5 (pentahapto) sandwich structure.
Robert Burns Woodward and Geoffrey Wilkinson deduced the structure based on its reactivity.[13] Independently Ernst Otto Fischer also came to the conclusion of the sandwich structure and started to synthesize other metallocenes such as nickelocene and cobaltocene.[14]
The structure of ferrocene was confirmed by NMR spectroscopy and X-ray crystallography.[11][15][16][17] Its distinctive "sandwich" structure led to an explosion of interest in compounds of d-block metals with hydrocarbons, and invigorated the development of the flourishing study of organometallic chemistry. In 1973 Fischer of the Technische Universität München and Wilkinson of Imperial College London shared a Nobel Prize for their work on metallocenes and other aspects of organometallic chemistry.[18]
Structure and bonding
The carbon–carbon bond distances are 1.40 Å within the five-membered rings, and the Fe–C bond distances are 2.04 Å. At room temperature down to 164K, X-ray crystallography points to the Cp rings being in a staggered conformation due to imposed molecular centrosymmetric symmetry in the monoclinic space group.[19] Below 110 K, ferrocene crystallizes in an orthorhombic crystal lattice in which the Cp rings are ordered and eclipsed.[20] It has been shown through gas phase electron diffraction[21] and computational studies[22] that in the gas phase the Cp rings are eclipsed. The point group of the staggered conformation is D5d and the point group of the eclipsed conformation is D5h.
The Cp rings rotate with a low barrier about the Cp(centroid)–Fe–Cp(centroid) axis, as observed by measurements on substituted derivatives of ferrocene using 1H and 13C nuclear magnetic resonance spectroscopy. For example, methylferrocene (CH3C5H4FeC5H5) exhibits a singlet for the C5H5 ring.[23]
In terms of bonding, the iron center in ferrocene is usually assigned to the +2 oxidation state, consistent with measurements using Mössbauer spectroscopy. Each cyclopentadienyl (Cp) ring is then allocated a single negative charge, bringing the number of π-electrons on each ring to six, and thus making them aromatic. These twelve electrons (six from each ring) are then shared with the metal via covalent bonding. When combined with the six d-electrons on Fe2+, the complex attains an 18-electron configuration.
Synthesis and handling properties
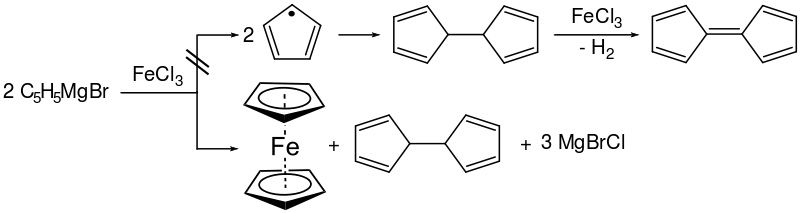
The first reported syntheses of ferrocene were nearly simultaneous. Pauson and Kealy synthesised ferrocene using iron(III) chloride and a Grignard reagent, cyclopentadienyl magnesium bromide. Iron(III) chloride is suspended in anhydrous diethyl ether and added to the Grignard reagent, which is prepared by reacting cyclopentadiene with magnesium and bromoethane in anhydrous benzene.[9] An iron(III) salt was chosen as they sought to couple the cyclopentadienyl moieties to form dihydrofulvalene and then fulvalene, but ferrocene was formed instead as the oxidative formation of dihydrofulvalene also produced iron(II) by reduction, which in turn reacts with the Grignard.

The other early synthesis of ferrocene was by Miller et al.,[10] who reacted metallic iron directly with gas-phase cyclopentadiene at elevated temperature.[24] An approach using iron pentacarbonyl was also reported.[25]
- Fe(CO)5 + 2 C5H6(g) → Fe(C5H5)2 + 5 CO(g) + H2(g)
More efficient preparative methods are generally a modification of the original transmetalation sequence using either commercially available sodium cyclopentadienide[26] or freshly cracked cyclopentadiene deprotonated with potassium hydroxide[27] and reacted with anhydrous iron(II) chloride in ethereal solvents. A modern modification of the Grignard approach is also known:
- 2 NaC5H5 + FeCl2 → Fe(C5H5)2 + 2 NaCl
- FeCl2·4H2O + 2 C5H6 + 2 KOH → Fe(C5H5)2 + 2 KCl + 6 H2O
- 2 C5H5MgBr + FeCl2 → Fe(C5H5)2 + 2 MgBrCl
Even some amine bases can be used for the deprotonation, though the reaction proceeds more slowly than when using stronger bases:[26]
- 2 C5H6 + 2 (CH3CH2)2NH + FeCl2 → Fe(C5H5)2 + 2 (CH3CH2)2NH2Cl
Direct transmetalation can also be used to prepare ferrocene from other metallocenes, such as manganocene:[28]
- FeCl2 + Mn(C5H5)2 → MnCl2 + Fe(C5H5)2

As expected for a symmetric, uncharged species, ferrocene is soluble in normal organic solvents, such as benzene, but is insoluble in water. Ferrocene is an air-stable orange solid that readily sublimes, especially upon heating in a vacuum. It is stable to temperatures as high as 400 °C.[29] The following table gives typical values of vapor pressure of ferrocene at different temperatures:[30]
| Pressure (Pa) | 1 | 10 | 100 |
|---|---|---|---|
| Temperature (K) | 298 | 323 | 353 |
Reactions
With electrophiles
Ferrocene undergoes many reactions characteristic of aromatic compounds, enabling the preparation of substituted derivatives. A common undergraduate experiment is the Friedel-Crafts reaction of ferrocene with acetic anhydride (or acetyl chloride) in the presence of phosphoric acid as a catalyst.

Protonation of ferrocene allows isolation of [[Cp2FeH+]PF6-.[31]
In the presence of aluminium chloride Me2NPCl2 and ferrocene react to give ferrocenyl dichlorophosphine,[32] whereas treatment with phenyldichlorophosphine under similar conditions forms P,P-diferrocenyl-P-phenyl phosphine.[33]
Ferrocene reacts with P4S10 forms a diferrocenyl-dithiadiphosphetane disulfide.[34]
Lithiation
Ferrocene reacts with butyllithium to give 1,1′-dilithioferrocene, which is a versatile nucleophile. Tert-Butyllithium produces monolithioferrocene.[35] Dilithioferrocene reacts S8, chlorophosphines, and chlorosilanes. The strained compounds undergo ring-opening polymerization.[36]

1,1′-bis(diphenylphosphino)ferrocene (dppf) is prepared from dilithioferrocene.
Redox chemistry – the ferrocenium ion
Ferrocene undergoes a one-electron oxidation at around 0.5 V versus a saturated calomel electrode (SCE). This reversible oxidation has been used as standard in electrochemistry as Fc+/Fc = 0.40 V versus the standard hydrogen electrode.[37] Ferrocenium tetrafluoroborate is a common reagent.[38]
Substituents on the cyclopentadienyl ligands alters the redox potential in the expected way: electron-withdrawing groups such as a carboxylic acid shift the potential in the anodic direction (i.e. made more positive), whereas electron-releasing groups such as methyl groups shift the potential in the cathodic direction (more negative). Thus, decamethylferrocene is much more easily oxidised than ferrocene and can even be oxidised to the corresponding dication.[39] Ferrocene is often used as an internal standard for calibrating redox potentials in non-aqueous electrochemistry.
Stereochemistry of substituted ferrocenes
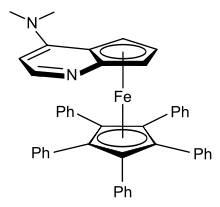
Disubstituted ferrocenes can exist as either 1,2-, 1,3- or 1,1′- isomers, none of which are interconvertible. Ferrocenes that are asymmetrically disubstituted on one ring are chiral – for example [CpFe(EtC5H3Me)]. This planar chirality arises despite no single atom being a stereogenic centre. The substituted ferrocene shown at right (a 4-(dimethylamino)pyridine derivative) has been shown to be effective when used for the kinetic resolution of racemic secondary alcohols.[40]
Applications of ferrocene and its derivatives
Ferrocene and its numerous derivatives have no large-scale applications, but have many niche uses that exploit the unusual structure (ligand scaffolds, pharmaceutical candidates), robustness (anti-knock formulations, precursors to materials), and redox (reagents and redox standards).
As a ligand scaffold
Chiral ferrocenyl phosphines are employed as ligands for transition-metal catalyzed reactions. Some of them have found industrial applications in the synthesis of pharmaceuticals and agrochemicals. For example, the diphosphine 1,1′-bis(diphenylphosphino)ferrocene (dppf) is a valued for palladium-coupling reactions and Josiphos ligand is useful for hydrogenation catalysis.[41] They are named after the technician who made the first one, Josi Puleo.[42][43]

Fuel additives
Ferrocene and its derivatives are antiknock agents used in the fuel for petrol engines; they are safer than tetraethyllead, previously used.[44] Petrol additive solutions containing ferrocene can be added to unleaded petrol to enable its use in vintage cars designed to run on leaded petrol.[45] The iron-containing deposits formed from ferrocene can form a conductive coating on the spark plug surfaces.
Pharmaceutical
Ferrocene derivatives have been investigated as drugs.[46] Only one drug has entered clinic trials, Ferroquine (7-chloro-N-(2-((dimethylamino)methyl)ferrocenyl)quinolin-4-amine), an antimalarial.[47][48].
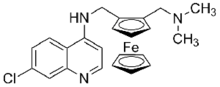
The anticancer activity of ferrocene derivatives was first investigated in the late 1970s, when derivatives bearing amine or amide groups were tested against lymphocytic leukemia.[49] Some ferrocenium salts exhibit anticancer activity, but no compound has seen evaluation in the clinic.[50] An experimental drug was reported which is a ferrocenyl version of tamoxifen.[51] The idea is that the tamoxifen will bind to the estrogen binding sites, resulting in cytotoxicity.[51][52]
Derivatives and variations
Ferrocene analogues can be prepared with variants of cyclopentadienyl. For example, bisindenyliron and bisfluorenyliron.[43]

Carbon atoms can be replaced by heteroatoms as illustrated by Fe(η5-C5Me5)(η5-P5) and Fe(η5-C5H5)(η5-C4H4N) ("azaferrocene"). Azaferrocene arises from decarbonylation of Fe(η5-C5H5)(CO)2(η1-pyrrole) in cyclohexane.[53] This compound on boiling under reflux in benzene is converted to ferrocene.[54]
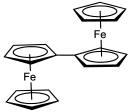
Because of the ease of substitution, many structurally unusual ferrocene derivatives have been prepared. For example, the penta(ferrocenyl)cyclopentadienyl ligand,[55] features a cyclopentadienyl anion derivatized with five ferrocene substituents.
cyclopentadienyl.png)
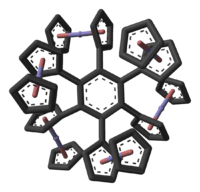
In hexaferrocenylbenzene, C6[(η5-C5H4)Fe(η5-C5H5)]6, all six positions on a benzene molecule have ferrocenyl substituents (R).[56] X-ray diffraction analysis of this compound confirms that the cyclopentadienyl ligands are not co-planar with the benzene core but have alternating dihedral angles of +30° and −80°. Due to steric crowding the ferrocenyls are slightly bent with angles of 177° and have elongated C-Fe bonds. The quaternary cyclopentadienyl carbon atoms are also pyramidalized. Also, the benzene core has a chair conformation with dihedral angles of 14° and displays bond length alternation between 142.7 pm and 141.1 pm, both indications of steric crowding of the substituents.
The synthesis of hexaferrocenylbenzene has been reported using Negishi coupling of hexaiodidobenzene and diferrocenylzinc, using tris(dibenzylideneacetone)dipalladium(0) as catalyst, in tetrahydrofuran:[56]
The yield is only 4%, which is further evidence consistent with substantial steric crowding around the arene core.
Materials chemistry
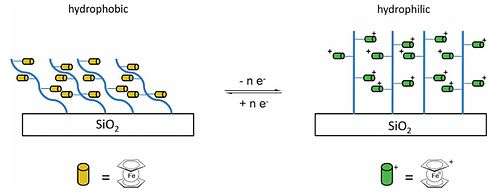
Ferrocene, a precursor to iron nanoparticles, can be used as a catalyst for the production of carbon nanotubes.[58] The vinylferrocene can be made by a Wittig reaction of the aldehyde, a phosphonium salt, and sodium hydroxide.[59] The vinyl ferrocene can be converted into a polymer (polyvinylferrocene, PVFc), a ferrocenyl version of polystyrene (the phenyl groups are replaced with ferrocenyl groups). Another polyferrocene which can be formed is poly(2-(methacryloyloxy)ethyl ferrocenecarboxylate), PFcMA. In addition to using organic polymer backbones, these pendant ferrocene units have been attached to inorganic backbones such as polysiloxanes, polyphosphazenes, and polyphosphinoboranes, (–PH(R)–BH2–)n, and the resulting materials exhibit unusual physical and electronic properties relating to the ferrocene / ferrocinium redox couple.[57] Both PVFc and PFcMA have been tethered onto silica wafers and the wettability measured when the polymer chains are uncharged and when the ferrocene moieties are oxidised to produce positively charged groups. The contact angle with water on the PFcMA-coated wafers was 70° smaller following oxidation, while in the case of PVFc the decrease was 30°, and the switching of wettability is reversible. In the PFcMA case, the effect of lengthening the chains and hence introducing more ferrocene groups is significantly larger reductions in the contact angle upon oxidation.[57][60]
See also
References
- ↑ "Ferrocene(102-54-5)". Retrieved 3 February 2010.
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 3.258. ISBN 0-8493-0486-5.
- ↑ "FERROCENE_msds".
- ↑ "Ferrocene MSDS". ScienceLab.
- ↑ "Material Safety Data Sheet. Ferrocene. MSDS# 03388. Section" (PDF). Northwest Missouri State University.
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0205". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Federman Neto, Alberto; Pelegrino, Alessandra Caramori; Darin, Vitor Andre (2004). "Ferrocene: 50 Years of Transition Metal Organometallic Chemistry – From Organic and Inorganic to Supramolecular Chemistry". ChemInform. 35 (43). doi:10.1002/chin.200443242.
- ↑ Pauson, P. L. (2001). "Ferrocene-how it all began". J. Organomet. Chem. 637-639: 3–6. doi:10.1016/S0022-328X(01)01126-3.
- 1 2 3 Kealy, T. J.; Pauson, P. L. (1951). "A New Type of Organo-Iron Compound". Nature. 168 (4285): 1039. Bibcode:1951Natur.168.1039K. doi:10.1038/1681039b0.
- 1 2 3 Miller, S. A.; Tebboth, J. A.; Tremaine, J. F. (1952). "114. Dicyclopentadienyliron". J. Chem. Soc.: 632–635. doi:10.1039/JR9520000632.
- 1 2 Laszlo, Pierre; Hoffmann, Roald (2000). "Ferrocene: Ironclad History or Rashomon Tale?". Angew. Chem. Int. Ed. 39 (1): 123–124. doi:10.1002/(SICI)1521-3773(20000103)39:1<123::AID-ANIE123>3.0.CO;2-Z. PMID 10649350.
- ↑ Werner, H (2012). "At Least 60 Years of Ferrocene: The Discovery and Rediscovery of the Sandwich Complexes". Angew. Chem. Int. Ed. 51: 6052–6058. doi:10.1002/anie.201201598.
- ↑ Wilkinson, G.; Rosenblum, M.; Whiting, M. C.; Woodward, R. B. (1952). "The Structure of Iron Bis-Cyclopentadienyl". J. Am. Chem. Soc. 74 (8): 2125–2126. doi:10.1021/ja01128a527.
- ↑ Fischer, E. O.; Pfab, W. (1952). "Zur Kristallstruktur der Di-Cyclopentadienyl-Verbindungen des zweiwertigen Eisens, Kobalts und Nickels" [On the crystal structure of the bis-cyclopentadienyl compounds of divalent iron, cobalt and nickel]. Zeitschrift für Naturforschung B. 7: 377–379.
- ↑ Dunitz, J. D.; Orgel, L. E. (1953). "Bis-Cyclopentadienyl – A Molecular Sandwich". Nature. 171 (4342): 121–122. Bibcode:1953Natur.171..121D. doi:10.1038/171121a0.
- ↑ Dunitz, J.; Orgel, L.; Rich, A. (1956). "The crystal structure of ferrocene". Acta Crystallogr. 9 (4): 373–375. doi:10.1107/S0365110X56001091.
- ↑ Eiland, P. F.; Pepinsky, R. (1952). "X-ray examination of iron biscyclopentadienyl". J. Am. Chem. Soc. 74 (19): 4971. doi:10.1021/ja01139a527.
- ↑ "Press Release: The Nobel Prize in Chemistry 1973". The Royal Swedish Academy of Sciences. 1973.
- ↑ Eiland, Philip Frank; Pepinsky, Ray (1952-10-01). "X-RAY EXAMINATION OF IRON BISCYCLOPENTADIENYL". Journal of the American Chemical Society. 74 (19): 4971–4971. doi:10.1021/ja01139a527. ISSN 0002-7863.
- ↑ Seiler, P.; Dunitz, J. D. (1982-06-15). "Low-temperature crystallization of orthorhombic ferrocene: structure analysis at 98 K". Acta Crystallographica Section B. 38 (6): 1741–1745. doi:10.1107/s0567740882007080. ISSN 0567-7408.
- ↑ Haaland, A.; Nilsson, J. E. (1968). "The Determination of Barriers to Internal Rotation by Means of Electron Diffraction. Ferrocene and Ruthenocene". Acta Chem. Scand. 22: 2653–2670. doi:10.3891/acta.chem.scand.22-2653.
- ↑ Coriani, Sonia; Haaland, Arne; Helgaker, Trygve; Jørgensen, Poul (2006). "The Equilibrium Structure of Ferrocene". ChemPhysChem. 7: 245–249. doi:10.1002/cphc.200500339.
- ↑ Abel, E. W.; Long, N. J.; Orrell, K. G.; Osborne, A. G.; Sik, V. (1991). "Dynamic NMR studies of ring rotation in substituted ferrocenes and ruthenocenes". J. Org. Chem. 403: 195–208. doi:10.1016/0022-328X(91)83100-I.
- ↑ Wilkinson, G.; Pauson, P. L.; Cotton, F. A. (1954). "Bis-cyclopentadienyl Compounds of Nickel and Cobalt". J. Am. Chem. Soc. 76 (7): 1970. doi:10.1021/ja01636a080.
- ↑ Wilkinson, G.; Cotton, F. A. (1959). "Cyclopentadienyl and Arene Metal Compounds". Prog. Inorg. Chem. 1: 1–124. doi:10.1002/978-0-470-16602-4.ch1. ISBN 978-0-470-16602-4.
- 1 2 Geoffrey Wilkinson (1963). "Ferrocene". Organic Syntheses. ; Collective Volume, 4, p. 473
- ↑ Jolly, W. L. (1970). The Synthesis and Characterization of Inorganic Compounds. New Jersey: Prentice-Hall.
- ↑ Wilkinson, G.; Cotton, F. A.; Birmingham, J. M. (1956). "On manganese cyclopentadienide and some chemical reactions of neutral bis-cyclopentadienyl metal compounds". J. Inorg. Nucl. Chem. 2 (2): 95. doi:10.1016/0022-1902(56)80004-3.
- ↑ Solomons, Graham; Fryhle, Craig (2006). Organic Chemistry (9th ed.). USA: John Wiley & Sons.
- ↑ Monte, Manuel J. S.; Santos, Luís M. N. B. F.; Fulem, Michal; Fonseca, José M. S.; Sousa, Carlos A. D. (2006). "New Static Apparatus and Vapor Pressure of Reference Materials: Naphthalene, Benzoic Acid, Benzophenone, and Ferrocene". J. Chem. Eng. Data. 51 (2): 757. doi:10.1021/je050502y.
- ↑ Malischewski, Moritz; Seppelt, Konrad; Sutter, Jörg; Heinemann, Frank W.; Dittrich, Birger; Meyer, Karsten (2017-09-19). "Protonation of Ferrocene: A Low-Temperature X-ray Diffraction Study of [Cp2FeH](PF6) Reveals an Iron-Bound Hydrido Ligand". Angewandte Chemie International Edition. 56: 13372–13376. doi:10.1002/anie.201704854.
- ↑ Knox, G. R.; Pauson, P. L.; Willison, D. (1992). "Ferrocene derivatives. 27. Ferrocenyldimethylphosphine". Organometallics. 11 (8): 2930–2933. doi:10.1021/om00044a038.
- ↑ Sollott, G. P.; Mertwoy, H. E.; Portnoy, S.; Snead, J. L. (1963). "Unsymmetrical Tertiary Phosphines of Ferrocene by Friedel–Crafts Reactions. I. Ferrocenylphenylphosphines". J. Org. Chem. 28 (4): 1090–1092. doi:10.1021/jo01039a055.
- ↑ Mark R. St. J. Foreman, Alexandra M. Z. Slawin, J. Derek Woollins (1996). "2,4-Diferrocenyl-1,3-dithiadiphosphetane 2,4-disulfide; structure and reactions with catechols and [PtCl2(PR3)2](R = Et or Bun)". J. Chem. Soc., Dalton Trans.: 3653–3657. doi:10.1039/DT9960003653.
- ↑ Rebiere, F.; Samuel, O.; Kagan, H. B. (1990). "A convenient method for the preparation of monolithioferrocene". Tetrahedron Lett. 31 (22): 3121–3124. doi:10.1016/S0040-4039(00)94710-5.
- ↑ Herbert, David E.; Mayer, Ulrich F. J.; Manners, Ian (2007). "Strained Metallocenophanes and Related Organometallic Rings Containing pi-Hydrocarbon Ligands and Transition-Metal Centers". Angew. Chem. Int. Ed. 46 (27): 5060–5081. doi:10.1002/anie.200604409.
- ↑ C. E. Housecroft & A. G. Sharpe, Inorganic Chemistry 4th edition, 2012, p. 925.
- ↑ Connelly, N. G.; Geiger, W. E. (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ↑ Malischewski, M.; Adelhardt, M.; Sutter, J.; Meyer, K.; Seppelt, K. (2016-08-12). "Isolation and structural and electronic characterization of salts of the decamethylferrocene dication". Science. 353 (6300): 678–682. Bibcode:2016Sci...353..678M. doi:10.1126/science.aaf6362. ISSN 0036-8075. PMID 27516596.
- ↑ Ruble, J. C.; Latham, H. A.; Fu, G. C. (1997). "Effective Kinetic Resolution of Secondary Alcohols with a Planar-Chiral Analogue of 4-(dimethylamino)pyridine. Use of the Fe(C5Ph5) Group in Asymmetric Catalysis". J. Am. Chem. Soc. 119 (6): 1492–1493. doi:10.1021/ja963835b.
- 1 2 [3]H-U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer and A. Togni, Top. Catal ., 2002, 19, 3.
- ↑ Privileged Chiral Ligands and Catalysts Qi-Lin Zhou 2011
- 1 2 Stepnicka, Petr (2008). Ferrocenes: Ligands, Materials and Biomolecules. Hoboken, NJ: J. Wiley. ISBN 0-470-03585-4.
- ↑ "Application of fuel additives" (PDF). Archived from the original (PDF) on 2006-05-05.
- ↑ US 4104036, Chao, Tai S., "Iron-containing motor fuel compositions and method for using same", issued 1978-08-01
- ↑ Van Staveren, Dave R.; Metzler-Nolte, Nils (2004). "Bioorganometallic Chemistry of Ferrocene". Chem. Rev. 104: 5931–5986. doi:10.1021/cr0101510.
- ↑ Biot, C.; Nosten, F.; Fraisse, L.; Ter-Minassian, D.; Khalife, J.; Dive, D. (2011). "The antimalarial ferroquine: from bench to clinic". Parasite. 18 (3): 207–214. doi:10.1051/parasite/2011183207. ISSN 1252-607X. PMC 3671469. PMID 21894260.

- ↑ Roux, C.; Biot, C., "Ferrocene-based antimalarials", Future Med. Chem. 2012, 4, 783-797. doi:10.4155/fmc.12.26
- ↑ Ornelas, Catia. "Application of ferrocene and its derivatives in cancer research". New Journal of Chemistry. 35. doi:10.1039/c1nj20172g.
- ↑ Babin, V. N., et al., "Ferrocenes as potential anticancer drugs. Facts and hypotheses", Russ. Chem. Bull. 2014, volume 63, 2405-2422. doi:10.1007/s11172-014-0756-7
- 1 2 Top, S.; Vessières, A.; Leclercq, G.; Quivy, J.; Tang, J.; Vaissermann, J.; Huché, M.; Jaouen, G. (2003). "Synthesis, Biochemical Properties and Molecular Modelling Studies of Organometallic Specific Estrogen Receptor Modulators (SERMs), the Ferrocifens and Hydroxyferrocifens: Evidence for an Antiproliferative Effect of Hydroxyferrocifens on both Hormone-Dependent and Hormone-Independent Breast Cancer Cell Lines". Chem. Eur. J. 9 (21): 5223–36. doi:10.1002/chem.200305024. PMID 14613131.
- ↑ Ron Dagani (16 September 2002). "The Bio Side of Organometallics". Chemical and Engineering News. 80 (37): 23–29. doi:10.1021/cen-v080n037.p023.
- ↑ Zakrzewski, J.; Giannotti, Charles (1990). "An improved photochemical synthesis of azaferrocene". J. Organomet. Chem. 388: 175. doi:10.1016/0022-328X(90)85359-7.
- ↑ Efraty, Avi; Jubran, Nusrallah; Goldman, Alexander (1982). "Chemistry of some η5-pyrrolyl- and η1-N-pyrrolyliron complexes". Inorg. Chem. 21 (3): 868. doi:10.1021/ic00133a006.
- ↑ Yu, Y.; Bond, A. D.; Leonard, P. W.; Vollhardt, K. P. C.; Whitener, G. D. (2006). "Syntheses, Structures, and Reactivity of Radial Oligocyclopentadienyl Metal Complexes: Penta(ferrocenyl)cyclopentadienyl and Congeners". Angew. Chem. Int. Ed. 45 (11): 1794–1799. doi:10.1002/anie.200504047. PMID 16470902.
- 1 2 Yu, Yong; Bond, Andrew D.; Leonard, Philip W.; Lorenz, Ulrich J.; Timofeeva, Tatiana V.; Vollhardt, K. Peter C.; Whitener, Glenn D.; Yakovenko, Andrey A. (2006). "Hexaferrocenylbenzene". Chem. Commun. (24): 2572–2574. doi:10.1039/b604844g. PMID 16779481.
- 1 2 3 Pietschnig, Rudolf (2016). "Polymers with pendant ferrocenes". Chem. Soc. Rev. 45: 5216–5231. doi:10.1039/C6CS00196C.
- ↑ Conroya, Devin; Moisalab, Anna; Cardosoa, Silvana; Windleb, Alan; Davidson, John (2010). "Carbon nanotube reactor: Ferrocene decomposition, iron particle growth, nanotube aggregation and scale-up". Chem. Eng. Sci. 65 (10): 2965–2977. doi:10.1016/j.ces.2010.01.019.
- ↑ Liu, Wan-yi; Xu, Qi-hai; Ma, Yong-xiang; Liang, Yong-min; Dong, Ning-li; Guan, De-peng (2001). "Solvent-free synthesis of ferrocenylethene derivatives". J. Organomet. Chem. 625: 128–132. doi:10.1016/S0022-328X(00)00927-X.
- ↑ Elbert, J.; Gallei, M.; Rüttiger, C.; Brunsen, A.; Didzoleit, H.; Stühn, B.; Rehahn, M. (2013). "Ferrocene Polymers for Switchable Surface Wettability". Organometallics. 32 (20): 5873–5878. doi:10.1021/om400468p.
External links
| Wikimedia Commons has media related to Ferrocene. |
- Ferrocene at The Periodic Table of Videos (University of Nottingham)
- NIOSH Pocket Guide to Chemical Hazards (Centers for Disease Control and Prevention)