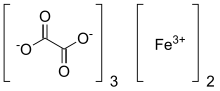
Ferric oxalate
 | |
| Names | |
|---|---|
| Systematic IUPAC name
iron(3+) ethanedioate (2:3) | |
| Other names
Iron(III) oxalate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.019.047 |
| EC Number | 220-951-7 |
PubChem CID |
|
| |
| |
| Properties | |
| C6Fe2O12 | |
| Molar mass | 375.747 g/mol |
| Appearance | Pale yellow solid (anhydrous) Lime green solid (hexahydrate) |
| Odor | odorless |
| slightly soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ferric oxalate, also known as iron(III) oxalate, is a chemical compound composed of ferric ions and oxalate ligands; it may also be regarded as the ferric salt of oxalic acid. The anhydrous material is pale yellow; however, it may be hydrated to form Fe2(C2O4)3·6H2O which is bright green in colour
Like many oxalates, ferric oxalate has been investigated as a short term treatment for dentin hypersensitivity.[1] It is used in certain toothpaste formulations; however, its effectiveness has been questioned.[2]
It is used as the light-sensitive element in the Kallitype photographic printing process; and the platinotype process Platinum/Palladium Printing.
See also
A number of other iron oxalates are known:-
References
- ↑ Gillam, D. G.; Newman, H. N.; Davies, E. H.; Bulman, J. S.; Troullos, E. S.; Curro, F. A. "Clinical evaluation of ferric oxalate in relieving dentine hypersensitivity". Journal of Oral Rehabilitation. 31 (3): 245–250. doi:10.1046/j.0305-182X.2003.01230.x.
- ↑ Cunha-Cruz, J.; Stout, J. R.; Heaton, L. J.; Wataha, J. C. (29 December 2010). "Dentin Hypersensitivity and Oxalates: a Systematic Review". Journal of Dental Research. 90 (3): 304–310. doi:10.1177/0022034510389179. PMC 3144108.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.