Iron(II,III) sulfide
| Names | |
|---|---|
| Other names
Ferrous-ferric sulfide, greigite | |
| Identifiers | |
| Properties | |
| FeS·Fe2S3 | |
| Molar mass | 295.805 |
| Appearance | Bluish-black, pinkish |
| Density | 4.049 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron(II,III) sulfide is a blue-black (sometimes pinkish) chemical compound of iron and sulfur with formula Fe3S4 or FeS·Fe2S3, which is much similar to iron(II,III) oxide. It occurs naturally as the sulfide mineral greigite and is magnetic. It is a bio-mineral produced by and found in magnetotactic bacteria. It is a mixed valence compound, featuring both Fe2+ and Fe3+ centers, in 1:2 ratio.
Crystal structure
The crystallographic unit cell is cubic, with space group Fd3m. The S anions form a cubic close-packed lattice, and the Fe cations occupy both tetrahedral and octahedral sites.[1][2]
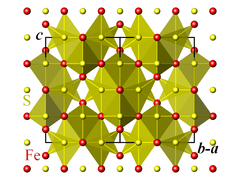
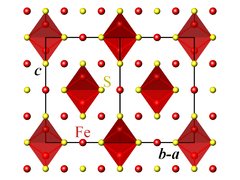
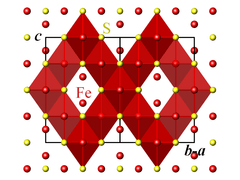
Magnetic and electronic properties
Like the related oxide magnetite (Fe3O4), iron(II,III) sulfide is ferrimagnetic, with the spin magnetic moments of the Fe cations in the tetrahedral sites oriented in the opposite direction as those in the octahedral sites, and a net magnetization. Both metal sites have high spin quantum numbers. The electronic structure of greigite is that of a half metal.[3][4]
References
- ↑ Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (1990). "Greigite". Handbook of Mineralogy (PDF). I (Elements, Sulfides, Sulfosalts). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209708. Retrieved December 5, 2011.
- ↑ Vaughan, D. J.; Craig, J. R. “Mineral Chemistry of Metal Sulfides" Cambridge University Press, Cambridge: 1978. ISBN 0-521-21489-0.
- ↑ Devey, Anthony; Grau-Crespo, R.; De Leeuw, N.H. (2009). "Electronic and magnetic structure of Fe3S4: GGA+U investigation". Physical Review B. 79 (19): 195126. Bibcode:2009PhRvB..79s5126D. doi:10.1103/PhysRevB.79.195126.
- ↑ Wang, Jun; Cao, Shi-He; Wu, Wei; Zhao, Guo-Meng (2011). "The Curie temperature and magnetic exchange energy in half-metallic greigite Fe3S4". Physica Scripta. 83 (4): 045702. Bibcode:2011PhyS...83d5702W. doi:10.1088/0031-8949/83/04/045702.
Further reading
- Mann, Stephen; Sparks, Nicholas H. C.; Frankel, Richard B.; Bazylinski, Dennis A.; Jannasch, Holger W. (1990). "Biomineralization of ferrimagnetic greigite (Fe3S4) and iron pyrite (FeS2) in a magnetotactic bacterium". Nature. 343 (6255): 258. Bibcode:1990Natur.343..258M. doi:10.1038/343258a0.
- Roberts, A (1995). "Magnetic properties of sedimentary greigite (Fe3S4)". Earth and Planetary Science Letters. 134: 227. Bibcode:1995E&PSL.134..227R. doi:10.1016/0012-821X(95)00131-U.
- Heywood, B. R.; Bazylinski, D. A.; Garratt-Reed, A.; Mann, S.; Frankel, R. B. (1990). "Controlled biosynthesis of greigite (Fe3S4) in magnetotactic bacteria". Naturwissenschaften. 77: 536. Bibcode:1990NW.....77..536H. doi:10.1007/BF01139266.
- Reynolds, R. L.; Tuttle, M. L.; Rice, C. A.; Fishman, N. S.; Karachewski, J. A.; Sherman, D. M. (1994). "Magnetization and geochemistry of greigite-bearing Cretaceous strata, North Slope basin, Alaska". American Journal of Science. 294: 485. doi:10.2475/ajs.294.4.485.
- Snowball, Ian; Thompson, Roy (1988). "The occurrence of Greigite in sediments from Loch Lomond". Journal of Quaternary Science. 3: 121. Bibcode:1988JQS.....3..121S. doi:10.1002/jqs.3390030203.
- Stanjek, H.; Fassbinder, J. W. E.; Vali, H.; Wagele, H.; Graf, W. (1994). "Evidence of biogenic greigite (ferrimagnetic Fe3S4) in soil". European Journal of Soil Science. 45: 97. doi:10.1111/j.1365-2389.1994.tb00490.x.
- Hoffmann, V (1992). "Greigite (Fe3S4): magnetic properties and first domain observations". Physics of the Earth and Planetary Interiors. 70: 288. Bibcode:1992PEPI...70..288H. doi:10.1016/0031-9201(92)90195-2.
- Fassbinder, Jörg W. E.; Stanjek, Helge (1994). "Magnetic properties of biogenic soil greigite (Fe3S4)". Geophysical Research Letters. 21: 2349. Bibcode:1994GeoRL..21.2349F. doi:10.1029/94GL02506.