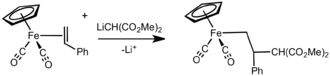Cyclopentadienyliron dicarbonyl dimer
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
Bis(cyclopentadienyl)tetracarbonyl-diiron, Di(cyclopentadienyl)tetracarbonyl-diiron, Bis(dicarbonylcyclopentadienyliron) | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.032.057 |
PubChem CID |
|
| |
| |
| Properties | |
| C14H10Fe2O4 | |
| Molar mass | 353.925 g/mol |
| Appearance | Dark purple crystals |
| Density | 1.77 g/cm3, solid |
| Melting point | 194 °C (381 °F; 467 K) |
| Boiling point | decomposition |
| insoluble | |
| Solubility in other solvents | benzene, THF, chlorocarbons |
| Structure | |
| distorted octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | CO source |
| R-phrases (outdated) | 20/22 |
| S-phrases (outdated) | 36/37 |
| Related compounds | |
Related compounds |
Fe(C5H5)2 Fe(CO)5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyclopentadienyliron dicarbonyl dimer is an organometallic compound with the formula (η5-C5H5)2Fe2(CO)4, also abbreviated Cp2Fe2(CO)4. It is called Fp2 or "fip dimer." It is a dark reddish-purple crystalline solid, which is readily soluble in moderately polar organic solvents such as chloroform and pyridine, but less soluble in carbon tetrachloride and carbon disulfide. Cp2Fe2(CO)4 is insoluble in but stable toward water.
Structure
In solution, Cp2Fe2(CO)4 can be considered a dimeric half-sandwich complex. It exists in three isomeric forms: cis, trans, and unbridged. These isomeric forms are distinguished by the position of the ligands. Cis and trans differ in the relative position of C5H5 (Cp) ligands. For both the cis and trans isomers, two CO ligands are terminal whereas the other two CO ligands bridge between the iron atoms. However, in the unbridged isomer, no ligands bridge between iron atoms — the metals are held together only by the Fe–Fe bond. Cis and trans isomers are the more abundant.
In solution, the three isomers interconvert. The phenomenon of rapidly interconverting structures is called fluxionality. The fluxional process for cyclopentadienyliron dicarbonyl dimer is so fast that only an averaged, single signal is observed in 1H NMR spectrum. However, the fluxional process is not fast enough to produce averaging in the IR spectrum. Thus, three absorptions are seen for each isomer. The νCO bands for bridging CO ligands are around 1780 cm−1 whereas νCO bands for terminal CO ligands are about 1980 cm−1.[1]
The solid-state molecular structure of both cis and trans isomers have been analyzed by X-ray and neutron diffraction. The Fe–Fe separation and the Fe–C bond lengths are the same in the Fe2C2 rhomboids, an exactly planar Fe2C2 four-membered ring in the trans isomer versus a folded rhomboid in cis with an angle of 164°, and significant distortions in the Cp ring of the trans isomer reflecting different Cp orbital populations.[2] Although older textbooks show the two iron atoms bonded to each other, theoretical analyses indicated the absence of a direct Fe–Fe bond.[3]
Synthesis
Cp2Fe2(CO)4 was first prepared by the same method employed today: the reaction of iron pentacarbonyl and dicyclopentadiene.[4]
- 2 Fe(CO)5 + C10H12 → (η5-C5H5)2Fe2(CO)4 + 6 CO + H2
In this preparation, dicyclopentadiene cracks to give cyclopentadiene, which reacts with Fe(CO)5 with loss of CO. Thereafter, the pathways for the photochemical and thermal routes differ subtly but both entail formation of a hydride intermediate.[2] The method is used in the teaching laboratory.[1]
Reactions
Although of no major commercial value, Fp2 is a workhorse in organometallic chemistry because it is inexpensive and FpX derivatives are rugged (X = halide, organyl).
"Fp−"
Reductive cleavage of the Cp2Fe2(CO)4 produces derivatives formally derived from the cyclopentadienyliron dicarbonyl anion, [CpFe(CO)2]− or called Fp−, which is assumed to exist as a tight ion pair. A typical reductant is sodium metal or sodium amalgam;[5] NaK alloy, and alkali metal trialkylborohydrides have been used. CpFe(CO)2]Na is a widely studied reagent since it is readily alkylated, acylated, or metalated by treatment with an appropriate electrophile.[6]
- [CpFe(CO)2]2 + 2 Na → 2 CpFe(CO)2Na
- [CpFe(CO)2]2 + 2 KBH(C2H5)3 → 2 CpFe(CO)2K + H2 + 2 B(C2H5)3
Treatment of NaFp with an alkyl halide (RX, X = Br, I) produces FeR(η5-C5H5)(CO)2
- CpFe(CO)2K + CH3I → CpFe(CO)2CH3 + KI
Fp2 can also be cleaved with alkali metals[7] and by electrochemical reduction.[8][9]
FpBr and FpI
Halogens oxidatively cleave Fp2 to give FpX (X = Cl, Br, I):
- [CpFe(CO)2]2 + X2 → 2 CpFe(CO)2X
One example is cyclopentadienyliron dicarbonyl iodide.
Fp(alkene)+
In the presence of halide anion acceptors such as AlBr3, FpX compounds (X = halide) react with alkenes to afford cationic alkene–Fp complexes. Salts of [Fp(isobutene)]+ are readily obtained by reaction of NaFp with methallyl chloride followed by protonolysis. This complex is a precursor to other cationic Fp–alkene complexes. The exchange process is facilitated by the loss of gaseous isobutene.[10]
Alkene–Fp complexes can also be prepared from Fp anion indirectly. Thus, hydride abstraction from Fp–alkyl compounds using triphenylmethyl hexafluorophosphate affords [Fp(isobutene)]+ complexes.
- FpNa + RCH2CH2I → FpCH2CH2R + NaI
- FpCH2CH2R + Ph3CPF6 → [Fp(CH2=CHR)+]PF−
6 + Ph3CH
Reaction of NaFp with an epoxide followed by acid-promoted dehydration also affords alkene complexes. Fp(alkene)+ are stable with respect to bromination, hydrogenation, and acetoxymercuration, but the alkene is easily released with sodium iodide in acetone or by warming with acetonitrile.[11]
The alkene ligand in these cations is activated toward attack by nucleophiles, opening the way to a number of carbon–carbon bond-forming reactions. Nucleophilic additions usually occur at the more substituted carbon. This regiochemistry is attributed to the greater positive charge density at this position. The regiocontrol is often modest. The addition of the nucleophile is completely stereoselective, occurring anti to the Fp group.
Fp-based cyclopropanation reagents
Fp-based reagents have been developed for cyclopropanations.[12] The key reagent is prepared from FpNa and has a good shelf-life, in contrast to typical Simmons-Smith intermediates and diazoalkanes.
- FpNa + ClCH2SCH3 → FpCH2SCH3 + NaCl
- FpCH2SCH3 + CH3I + NaBF4 → FpCH2S(CH3)2]BF4 + NaI
Use of [FpCH2S(CH3)2]BF4 does not require specialized conditions.
- Fp(CH2S+(CH3)2) BF−
4 + (Ph)2C=CH2 → 1,1-diphenylcyclopropane + ....
Ferric chloride is added to destroy any byproduct.
Photochemical reaction
Fp2 exhibits photochemistry.[13] Upon UV irradiation at 350 nm, it is reduced by 1-benzyl-1,4-dihydronicotinamide dimer, (BNA)2.[14]
- [CpFe(CO)2]2 + (BNA)2 → 2[CpFe(CO)2]− + 2BNA+
References
- 1 2 Girolami, G.; Rauchfuss, T.; Angelici, R. (1999). Synthesis and Technique in Inorganic Chemistry (3rd ed.). Sausalito, CA: University Science Books. pp. 171–180. ISBN 978-0-935702-48-4.
- 1 2 Wilkinson, G., ed. (1982). Comprehensive Organometallic Chemistry. 4. New York: Pergamon Press. pp. 513–613. ISBN 978-0-08-025269-8.
- ↑ Green, Jennifer C.; Green, Malcolm L. H.; Parkin, Gerard (2012). "The occurrence and representation of three-centre two-electron bonds in covalent inorganic compounds". Chem. Commun. 2012: 11481–11503. doi:10.1039/c2cc35304k.
- ↑ Piper, T. S.; Cotton, F. A.; Wilkinson, G. (1955). "Cyclopentadienyl–carbon monoxide and related compounds of some transitional metals". J. Inorg. Nuc. Chem. 1: 165–74. doi:10.1016/0022-1902(55)80053-X.
- ↑ Chang, T. C. T.; Rosenblum, M.; Simms, N. (1988). "Vinylation of Enolates with a Vinyl Cation Equivalent: trans-3-Methyl-2-Vinylcyclohexanone". Organic Syntheses. 66: 95. ; Collective Volume, 8, p. 479
- ↑ King, B. (1970). "Applications of Metal Carbonyl Anions in the Synthesis of Unusual Organometallic Compounds". Acc. Chem. Res. 3 (12): 417–427. doi:10.1021/ar50036a004.
- ↑ Ellis, J. E.; Flom, E. A. (1975). "The Chemistry of Metal Carbonyl Anions: III. Sodium-Potassium Alloy: An Efficient Reagent for the Production of Metal Carbonyl Anions". J. Organomet. Chem. 99 (2): 263–268. doi:10.1016/S0022-328X(00)88455-7.
- ↑ Dessy, R. E.; King, R. B.; Waldrop, M. (1966). "Organometallic Electrochemistry. V. The Transition Series". J. Am. Chem. Soc. 88 (22): 5112–5117. doi:10.1021/ja00974a013.
- ↑ Dessy, R. E.; Weissman, P. M.; Pohl, R. L. (1966). "Organometallic Electrochemistry. VI. Electrochemical Scission of Metal-Metal Bonds". J. Am. Chem. Soc. 88 (22): 5117–5121. doi:10.1021/ja00974a014.
- ↑ Mark M. Turnbull (2001). "Dicarbonyl(cyclopentadienyl)(isobutene)iron Tetrafluoroborate". eEROS. doi:10.1002/047084289X.rd080.
- ↑ Pearson, A. J. (1994). Iron Compounds in Organic Synthesis. San Diego, CA: Academic Press. pp. 22–35. ISBN 978-0-12-548270-7.
- ↑ Mattson, M. N.; O'Connor, E. J.; Helquist, P. (1992). "Cyclopropanation Using an Iron-Containing Methylene Transfer Reagent: 1,1-Diphenylcyclopropane". Organic Syntheses. 70: 177. ; Collective Volume, 9, p. 372
- ↑ Wrighton, M. (1974). "Photochemistry of Metal Carbonyls". Chem. Rev. 74 (4): 401–430. doi:10.1021/cr60290a001.
- ↑ Fukuzumi, S.; Ohkubo, K.; Fujitsuka, M.; Ito, O.; Teichmann, M. C.; Maisonhaute, E.; Amatore, C. (2001). "Photochemical Generation of Cyclopentadienyliron Dicarbonyl Anion by a Nicotinamide Adenine Dinucleotide Dimer Analogue". Inorg. Chem. 40 (6): 1213–1219. doi:10.1021/ic0009627.
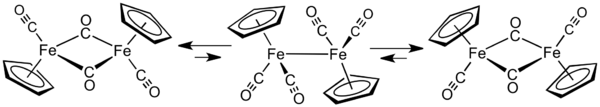
cation.png)
exchange.png)