Iron(III) bromide
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Iron(III) bromide | |
| Other names
Ferric bromide Iron tribromide tribromoiron | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.069 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| FeBr3 | |
| Molar mass | 295.56 g mol−1 |
| Appearance | brown solid |
| Odor | odorless |
| Density | 4.50 g cm−3 |
| Melting point | 200 °C (392 °F; 473 K) (decomposes) |
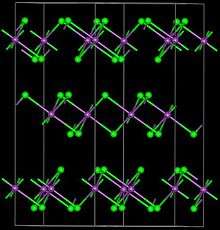
| Structure | |
| Trigonal, hR24 | |
| R-3, No. 148 | |
| Hazards | |
| Main hazards | corrosive |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S26 S37/39 |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iron(III) bromide is the chemical compound with the formula FeBr3. Also known as ferric bromide, this red-brown odorless compound is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It dissolves in water to give acidic solutions.
Structure, synthesis and basic properties
FeBr3 forms a polymeric structure featuring six-coordinate, octahedral Fe centers.[1] Although inexpensively available commercially, FeBr3 can be prepared by treatment of iron metal with bromine:
- 2 Fe + 3 Br2 → 2 FeBr3
Above 200 °C, FeBr3 decomposes to ferrous bromide:
- 2FeBr3 → 2FeBr2 + Br2
Iron(III) chloride is considerably more stable, reflecting the greater oxidizing power of chlorine. FeI3 is not stable, as iron(III) will oxidize iodide ions.
Uses
Ferric bromide is occasional use as an oxidant in organic chemistry, e.g. for the conversion of alcohols to ketones. It is used as a Lewis acidic catalyst for bromination of aromatic compounds. For the latter applications, it is often generated in situ.[2]
See also
- Iron(II) bromide, the lower bromide of iron
References
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Drapeau, Martin Pichette; Lafantaisie, Mathieu; Ollevier, Thierry (2013). "Iron(III) bromide". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn01568.
