Potassium ferrioxalate
_trihydrate_in_vial.jpg) | |
 | |
| Names | |
|---|---|
| IUPAC name
Potassium iron(III) oxalate | |
| Other names
potassium ferrioxalate potassium trisoxalatoferrate(III) | |
| Identifiers | |
| ECHA InfoCard | 100.035.398 |
| Properties | |
| K 3[Fe(C 2O 4) 3] (anhydrous) K 3[Fe( C 2O 4)3]·3H 2O (trihydrate) | |
| Molar mass | 437.20 g/mol (anhydrous) 491.25 g/mol (trihydrate) |
| Appearance | emerald green hydrated crystals |
| Density | 2.13 g/cm3 |
| Melting point | 230 °C (446 °F; 503 K) the trihydrate loses 3H2O at 113 °C[1] |
| Structure | |
| octahedral | |
| 0 D | |
| Hazards | |
| Main hazards | Corrosive. Eye, respiratory and skin irritant. |
| R-phrases (outdated) | R20, R21, R22, R34, R36/37/38 |
| Related compounds | |
Other anions |
Sodium ferrioxalate |
Related compounds |
Iron(II) oxalate Iron(III) oxalate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
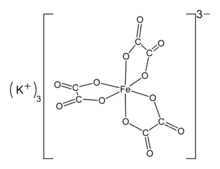
Potassium ferrioxalate, also known as potassium trisoxalatoferrate(III), is a chemical compound with the formula K
3[Fe(C
2O
4)
3], where iron is in the +3 oxidation state. It is an octahedral transition metal complex in which three bidentate oxalate ions are bound to an iron center. Potassium acts as a counterion, balancing the −3 charge of the complex. Crystals of the trihydrated form of the complex, K
3[Fe(C
2O
4)
3]·3H
2O, are emerald green in color. In solution, the salt dissociates to give the ferrioxalate anion, [Fe(C
2O
4)
3]3−, which appears fluorescent green in color. Potassium ferrioxalate is often used in chemical actinometry, i.e. the measure of light flux.
Preparation
_trihydrate.jpg)
The complex can be synthesized by the reaction between iron(III) sulfate, barium oxalate and potassium oxalate:[2]
- Fe
2(SO
4)
3 + 3 BaC
2O
4 + 3 K
2C
2O
4 → 2 K
3[Fe(C
2O
4)
3] + 3 BaSO
4
The reactants are combined in water, the solid precipitate of BaSO
4 is removed, and the green trihydrate crystallizes from the cooled solution.
Another common synthesis is reacting aqueous iron(III) chloride hexahydrate and potassium oxalate monohydrate.[3]
- FeCl
3·6H
2O + 3 K
2C
2O
4·H
2O → K
3[Fe(C
2O
4)
3]·3H
2O + 3 KCl + 6H
2O
Structure
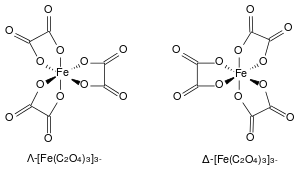
The ferrioxalate complex has D3 molecular symmetry, within which the iron center is octahedral. The six Fe–O bond distances all close to 2.0 Å[4] which indicates that the Fe(III) is high spin; as the low spin complex would display Jahn–Teller distortions. The ammonium and mixed sodium-potassium salts are isomorphous, as are related complexes with Al3+, Cr3+, and V3+.
The ferrioxalate complex displays helical chirality as it can form two non-superimposable geometries. In accordance with the IUPAC convention, the isomer with the left-handed screw axis is assigned the Greek symbol Λ (lambda). Its mirror image with the right-handed screw axis is given the Greek symbol Δ (delta).[5]
Reactions
Photoreduction
In solution, the ferrioxalate complex undergoes photoreduction. In this process, the complex absorbs a photon of light and subsequently decomposes to form Fe(C
2O
4)2−
2 and CO
2. The iron centre is reduced (gains an electron) from the +3 to the +2 oxidation state, while an oxalate ion is oxidised to carbon dioxide:
- 2 [Fe(C
2O
4)
3]3− + hν → 2 [Fe(C
2O
4)
2]2− + 2 CO
2 + C
2O2−
4[6]
Thermal decomposition
The trihydrate loses the three water molecules at the same time when heated at 113 °C.[1]
At 296 °C, the anhydrous salt decomposes into the iron(II) complex potassium ferrooxalate, potassium oxalate, and carbon dioxide:[1]
- 2 K
3[Fe(C
2O
4)
3] → 2 K
2[Fe(C
2O
4)
2] + K
2C
2O
4 + 2 CO
2
See also
A number of other iron oxalates are known
References
- 1 2 3 J. Ladriere (1992): "Mössbauer study on the thermal decomposition of potassium tris (oxalato) ferrate(III) trihydrate and bis (oxalato) ferrate(II) dihydrate". Hyperfine Interactions, volume 70, issue 1, pages 1095–1098. doi:10.1007/BF02397520
- ↑ Bailar, John C.; Jones, Eldon M. (1939). "Trioxalato Salts (Trioxalatoaluminiate, -ferriate, -chromiate, and -cobaltiate)". Inorg. Synth. 1: 35–38. doi:10.1002/9780470132326.ch13.
- ↑ Performed by Benjamin J. Abram, OSU lab manual, Dr. Richard Nafshun
- ↑ Junk, Peter C. (2005). "Supramolecular interactions in the X-ray crystal structure of potassium tris(oxalato)ferrate(III) trihydrate". J. Coord. Chem. 58 (4): 355–361. doi:10.1080/00958970512331334250.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Pozdnyakov, Ivan P.; Kel, Oksana V.; Plyusnin, Victor F.; Grivin, Vyacheslav P.; Bazhin, Nikolai M. (2008). "New Insight into Photochemistry of Ferrioxalate". J. Phys. Chem. A. 112 (36): 8316–8322. doi:10.1021/jp8040583.